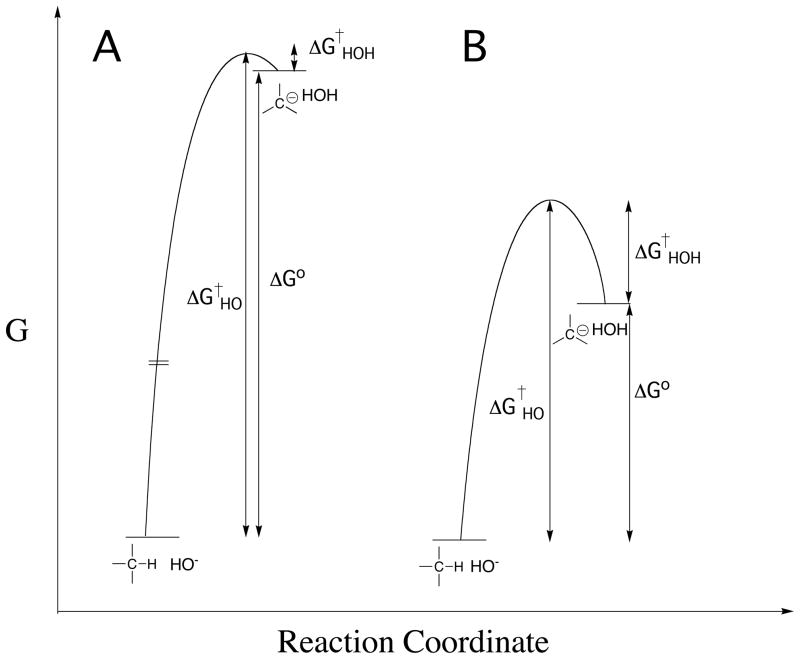
Figure 1.
Profiles that show the changes in free energy for deprotonation of weak carbon acids (eq 1) to form carbanion products. Note that the barrier to thermodynamically unfavorable proton transfer (ΔG†HO) is equal to the sum of the thermodynamic barriers to proton transfer (ΔG°) and the barrier to downhill protonation of the carbanion in the reverse direction (ΔG†HOH). (A) Strongly thermodynamically unfavorable proton transfer reaction, for which there is a limiting small barrier to protonation of the carbanion by water. (B) Thermodynamically unfavorable formation of a strongly resonance stabilized carbanion, for which there is a substantial kinetic barrier to protonation of the carbanion by water [61].