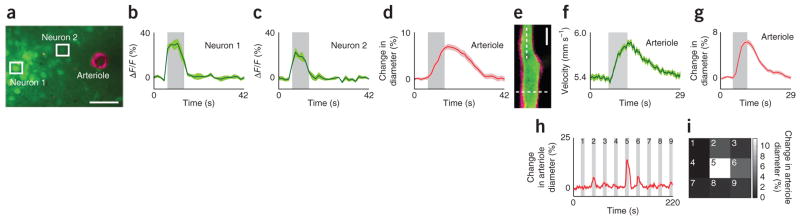
Figure 2.
Monitoring sensory stimulus–evoked responses in arterioles and adjacent neurons. (a–d) Simultaneous tracking of neuronal activation using the calcium dye Oregon Green 488 Bapta-1 acetoxymethyl ester (OGB-1 AM) and changes in arteriole diameter using Alexa Fluor 633 in layer 2/3 of the cat visual cortex. Image of an Alexa Fluor 633–labeled (magenta) penetrating arteriole and adjacent neuronal cell bodies labeled with OGB-1 AM (green) (a). Scale bar, 50 μm. Time courses (averages of 24 trials) of responses to drifting grating visual stimuli from neurons (b,c) and arteriole (d) marked in a. ΔF/F, relative change in fluorescence. (e–g) Measurement of red blood cell velocity and vessel diameter in an arteriole of the rat visual cortex. Imaged field of view (e) showing an arteriole labeled with Alexa Fluor 633 (magenta) and its lumen labeled with fluorescein dextran (green). Scale bar, 20 μm. Time courses (averages of 32 trials) of increases in velocity (f) and diameter (g) in response to drifting grating visual stimuli measured at locations marked by dotted lines in e. (h,i) Dilation of an Alexa Fluor 633–labeled arteriole in the cat visual cortex (h) in response to the presentation of visual stimuli in each of the nine locations (3 × 3 grid) of a stimulus display monitor (i). Average responses to five repeats of the entire visual stimulus sequence are plotted in h. The response to each stimulus position is plotted in the corresponding location in the 3 × 3 grid in which the luminance value represents the dilation magnitude. All gray bars, period of visual stimulation; error bands, s.e.m.