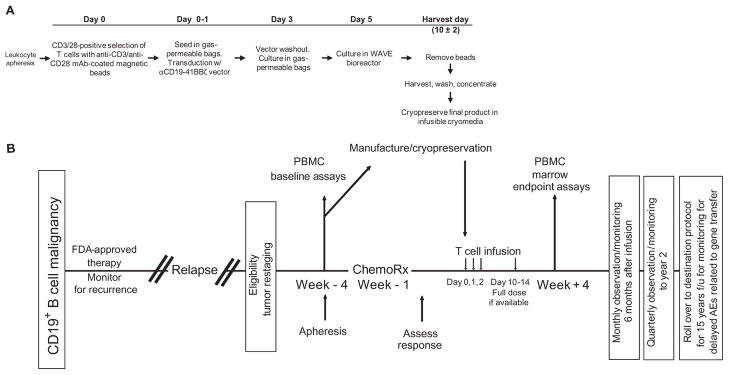
Fig. 1.
Schematic representation of the gene transfer vector and transgene, gene-modified T cell manufacturing, and clinical protocol design. (A) T cell manufacturing. Autologous cells were obtained via leukapheresis, and T cells were enriched by mononuclear cell elutriation, washed, and expanded by addition of anti-CD3/CD28–coated paramagnetic beads for positive selection and activation of T cells. Residual leukemic cells were depleted. The lentiviral vector was added at the time of cell activation and was washed out on day 3 after culture initiation. Cells were expanded on a rocking platform device (WAVE Bioreactor System) for 8 to 12 days. On the final day of culture, the beads were removed by passage over a magnetic field and the CART19 cells were harvested and cryopreserved in infusible medium. mAb, monoclonal antibody. (B) Clinical protocol design. Patients were given lymphodepleting chemotherapy as described, followed by CART19 infusion #1 by intravenous gravity flow drip over a period of 15 to 20 min. The infusion was given using a split-dose approach over 3 days (10, 30, and 60%) beginning 1 to 5 days after completion of chemotherapy. Endpoint assays were conducted on study week 4. At the conclusion of active monitoring, subjects were transferred to a destination protocol for long-term follow-up as per FDA guidance.