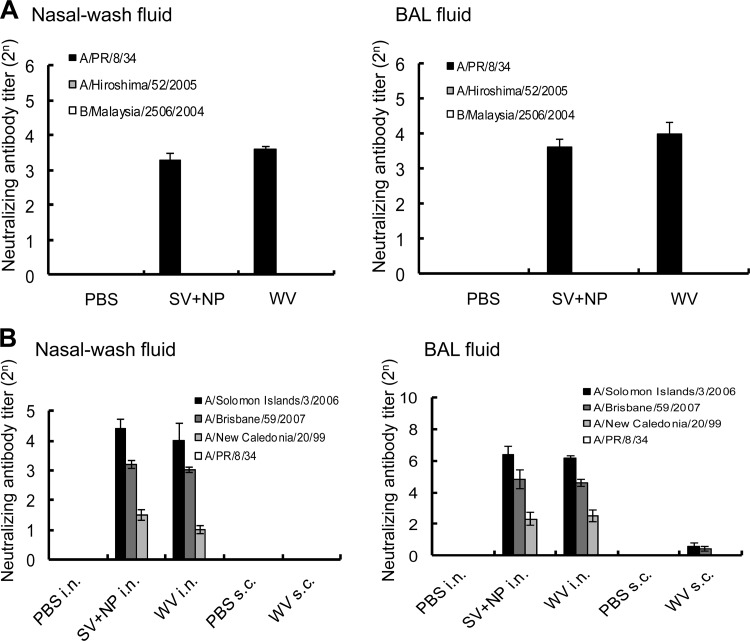
Fig 8.
Cross-reactive anti-influenza neutralizing antibody titer in the nasal wash and BAL fluids of mice intranasally immunized with A/PR/8/34 vaccines. (A) Groups of mice (50 per group) were inoculated twice intranasally with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/PR/8/34 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/PR/8/34 WV vaccine (WV). Fourteen days after the final immunization, the BAL and nasal wash fluids were harvested. Fluids from 5 mice were pooled, pretreated with RDE, and concentrated. The neutralizing antibody titers for A/PR/8/34 (H1N1), A/Hiroshima/52/2005 (H3N2), and B/Malaysia/2506/2005 in the concentrated samples were assessed. Bars represent the means ± standard deviations from 10 concentrated samples. (B) Anti-influenza neutralizing antibody titer for drift variants within a virus subtype in the nasal wash and BAL fluids of mice intranasally immunized with A/Solomon Islands/3/2006 vaccines. Groups of mice (50 per group) were inoculated twice intranasally with PBS, 100 μg of γ-PGA-NPs (NP), a mixture of 1 μg of A/Solomon islands/3/2006 SV vaccine and 100 μg of γ-PGA-NPs (SV+NP), or 1 μg of A/Solomon Islands/3/2006 WV vaccine (WV). Fourteen days after the final immunization, the BAL and nasal wash fluids were harvested. Fluids from 5 mice were pooled, pretreated with RDE, and concentrated. The neutralizing antibody titers for A/Solomon Islands/3/2006, A/Brisbane/59/2007, A/New Caledonia/20/99, and A/PR/8/34 were then assessed. Bars represent the means ± standard deviations from 10 concentrated samples.