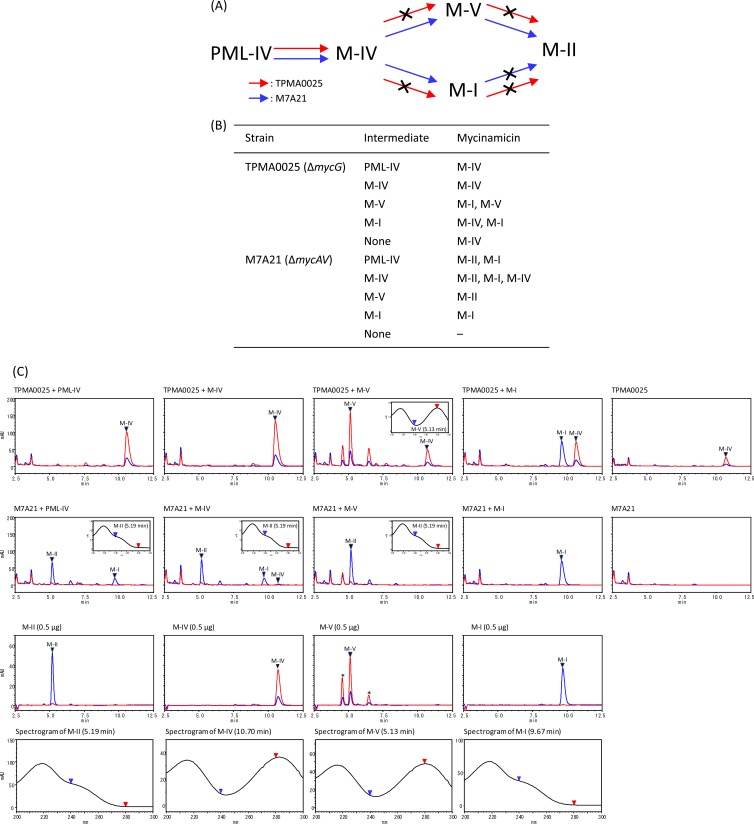
Fig 3.
(A) Mycinamicin biosynthetic pathway of the mycG disruption mutant TPMA0025 and the mycAV disruption mutant M7A21 based on a bioconversion study of mycinamicin biosynthetic intermediates (B). The crossed arrows in panel A represent the undetected pathways in the mutants. (B) Bioconversion of mycinamicin biosynthetic intermediates by TPMA0025 and M7A21. Mycinamicin intermediates (500 μg) were added to culture plates of TPMA0025 and M7A21, and the culture plates were incubated at 27°C for 8 days. Mycinamicins in the ethyl acetate (EtOAc) extracts from the culture plates fed mycinamicin biosynthetic intermediates were analyzed with HPLC culture plate fed M-IV (C). Trace amounts of M-IV were detected in M7A21 culture plate fed M-IV. (C) HPLC chromatograms of the EtOAc extract obtained from culture plates fed with mycinamicin intermediates (PML-IV, M-IV, M-V, and M-I) of TPMA0025 and M7A21 and UV spectrograms of M-II and M-V detected in the EtOAc extract. HPLC chromatograms of the mycinamicin intermediates are shown with UV spectrograms. The purity of M-V was approximately 70%; other compounds included in the M-V powder are shown with asterisks. HPLC conditions: column, ODS-80Tm (Tosoh); mobile phase, MeCN, 0.06% TFA (35:65); flow rate, 0.8 ml/min; UV wavelength, 200 to 300 nm. Blue line, at 240 nm; red line, at 280 nm.