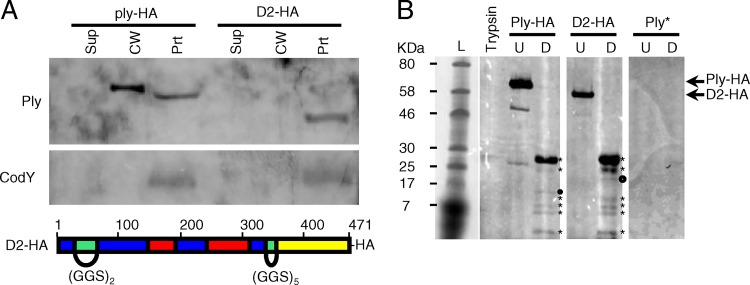
Fig 6.
Ply with domain 2 replacement does not localize to the cell wall. Domain 2 was replaced with flexible linkers comprised of GGS repeats of appropriate length to maintain the three-dimensional spatial orientation of the remaining domains, tagged with a C-terminal HA epitope, and crossed into the ply locus on the S. pneumoniae chromosome. (A) Top, the ply-HA and D2 strains were grown to mid-exponential phase, fractionated into supernatant, cell wall, and protoplast compartments, and assayed for the presence of Ply and CodY by Western blotting. Equal cell equivalents were loaded on the gel. Sup, culture supernatant; CW, cell wall; Prt, protoplast. Bottom, schematic of the D2 replacement mutant, color coded by domain structure as in Fig. 5: blue, domain 1; green, domain 2; red, domain 3; yellow, domain 4. (B) Limited proteolysis of Ply with the domain 2 linker replacement reveals a wild-type conformation. Ply-HA and D2-HA were purified from strains Ply-HA and D2, respectively, via immunoprecipitation. His-tagged Ply toxoid protein (Ply*) served as a negative control for the anti-HA antibody. Approximately equivalent amounts of each purified protein were incubated either alone (U) or in the presence of identical amounts of trypsin (D). Trypsin alone was incubated under the same conditions. Equal amounts of each reaction product were analyzed for the presence of HA-tagged trypsin-cleaved peptides by Western blotting. The proteolytic degradation products of Ply-HA and D2-HA (marked with asterisks) are nearly identical. The presence of a single unique product in each (marked with circles) suggests a local change in surface accessibility that simultaneously exposes one trypsin cleavage site while deleting or masking another.