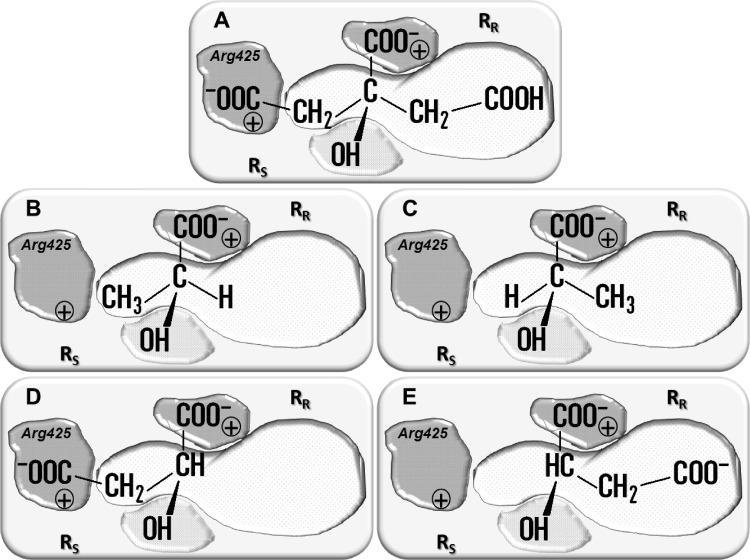
Fig 1.
Schematic models of the substrate binding pocket of CitP. Substrates depicted in the pocket are citrate (A), (S)-lactate (B), (R)-lactate (C), (S)-malate (D), and (R)-malate (E). The interactions between the carboxylate groups and the hydroxyl group of the substrate and the protein are indicated by gray surfaces, and the hydrophobic interaction site (RR) is indicated by a white surface. The RS and RR sites bind the side chains of the (S)- and (R)-enantiomers of monosubstituted 2-hydroxycarboxylates (HO-CHR-COO−), respectively. Residue Arg425, responsible for binding of a carboxylate in the R side chain, is indicated in the RR site when present.