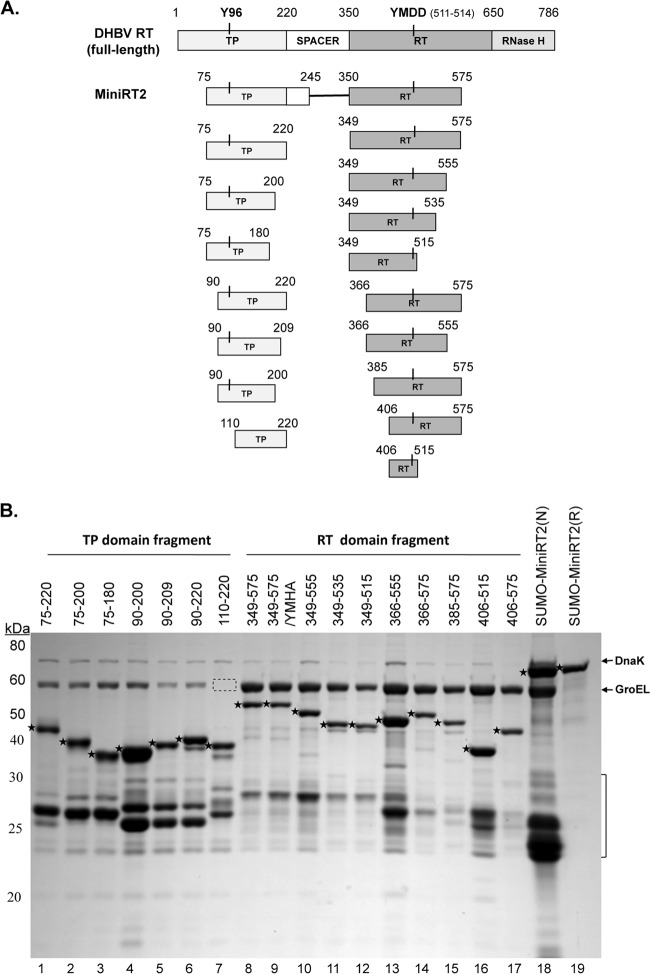
Fig 1.
Construction of DHBV RT proteins and individual TP and RT domains. (A) Schematic diagrams of the RT protein and individual TP and RT domain constructs. The top diagram depicts the full-length DHBV RT protein, with the primer Y residue (Y96) in the TP domain and the 511YMDD514 active site in the RT domain denoted. The boundaries (in amino acid positions) of the truncated MiniRT2 (second diagram) and the TP (left) and RT (right) domain constructs (below the MiniRT2 diagram) are indicated. (B) Purification of RT proteins and domains. The DHBV SUMO-MiniRT2 protein and the GST-tagged TP and RT domain constructs were expressed in Escherichia coli and purified by affinity methods as described in Materials and Methods. The GST-tagged TP and RT domains were purified under native conditions (lanes 1 to 17). SUMO-MiniRT2 (His tagged) was purified under either native (N, lane 18) or denaturing (and refolding [R], lane 19) conditions. The purified MiniRT2 protein and domains were analyzed by SDS-PAGE and Coomassie blue staining. The intact protein or domain species are denoted by the stars to the left of the corresponding bands. The copurifying bacterial chaperone proteins, DnaK and GroEL, are also indicated. The absence of GroEL in TP/110–220 (lane 7) is denoted by the dashed box. The protein molecular mass markers are indicated on the left in kDa. The bracket denotes the degradation products from the GST-tagged TP and RT domain constructs or SUMO-MiniRT2, consisting mostly of the GST or SUMO tag plus variable amounts of the TP or RT sequences remaining attached to the tag.