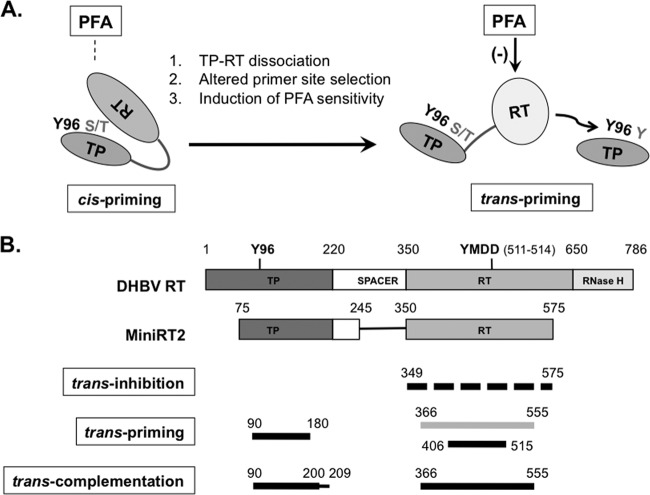
Fig 10.
Proposed RT conformational dynamics and TP and RT domain interactions in protein priming. (A) RT conformational changes following initiation of protein priming. The authentic primer site (Y96 in TP) and cryptic priming sites (S/T or Y in TP) are indicated. For clarity, the cryptic priming sites in the RT domain are omitted. The resistance (left) of cis-priming and sensitivity (right) of trans-priming to PFA inhibition are also indicated. The dissociation of cis-linked TP and RT domains upon cis-priming is depicted as an opening of the protein structure. The proposed conformational change in the RT domain is depicted as a change in the shape and shading of the RT domain. (B) Definition of TP and RT domain sequences required for trans-inhibition, trans-priming, and trans-complementation. The top two diagrams depict the domain structures of the full-length DHBV RT and MiniRT2, as explained in Fig. 1A. The dashed lines on the third diagram indicate that multiple sequences in the RT domain could function to inhibit priming in trans. The light shading in the fourth diagram signifies the fact that the longer RT domain construct (containing position 366 to 555) failed to serve as a primer, in trans, to be used by another RT domain but could prime from itself in cis (when provided with a functional TP domain). The thinner line in the fifth diagram (denoting residues 200 to 209) signifies that these TP sequences, while not essential for trans-complementation, nevertheless contribute substantially to the reaction. See the text for details.