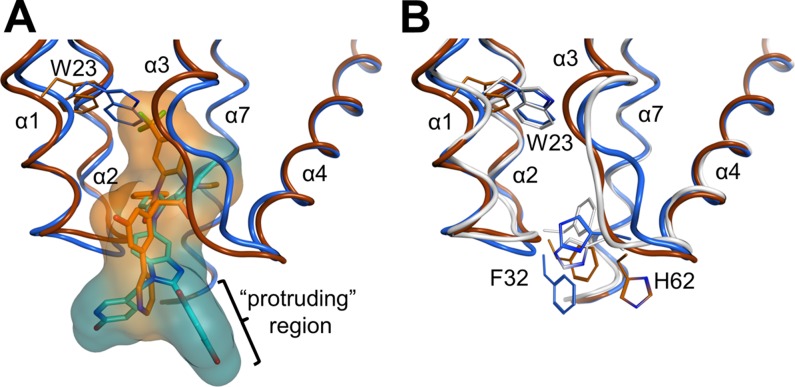
Fig 3.
Structural comparisons illustrating the differing effects of BD and BM inhibitor binding on the CANTD conformation. (A) Overlay of the BD 3 (orange) and BM 4 (blue) complex structures. van der Waals surfaces illustrate how BM 4 protrudes further into the solvent, whereas BD 3 binds more deeply and induces the formation of a larger pocket, primarily by shifting CA-NC helices 1 and 2 and the Trp23 side chain. The “protruding region,” or the substituents on the inhibitors that extend outside the pocket, is indicated (see the text). (B) Overlay of the apo (white)-, BD 3 (orange)-, and BM 4 (blue)-bound CANTD structures detailing shifts of backbones and key residues. The bound inhibitors have been removed for clarity. Residues 1 to 146 of the stabilized hexameric CA structure (PDB ID 3H47) (29) were used as the reference apo structure. Residues of helices 1 and 2 and highly flexible portions of CANTD were omitted from the superposition calculations, as described in Materials and Methods.