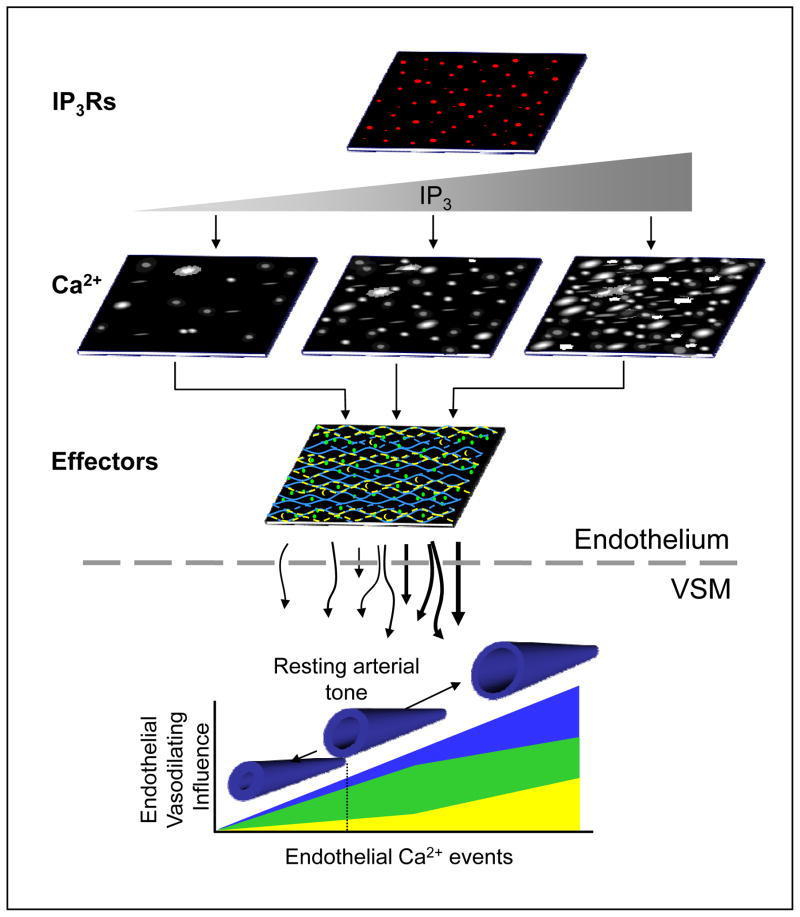
Figure 1.
Conceptual model of dynamic Ca2+-effector coupling in the endothelium and its real-time regulation of arterial tone. It is proposed that a discrete scaffold of IP3Rs within the endoplasmic reticulum of the vascular intima establishes an intrinsic mode of dynamic Ca2+ signals. Relative shifts in IP3 production define new profiles of dynamic signaling with respect to the number of sites as well as event amplitude, frequency, duration and spatial spread. The distribution and density of specific Ca2+-dependent effectors within the endothelium, including those that are tightly coupled to local Ca2+ origination sites (blue) such as KCa3.1 channels, and those that are more peripherally or widely distributed (yellow and green) such as KCa2.3 channels and eNOS, determine the ultimate level of vasodilating influence communicated across the internal elastic lamina (IEL) to the vascular smooth muscle (VSM) as well as the predominating mechanism solicited (i.e. NO vs. hyperpolarization). This suggests a basal dynamic Ca2+ signaling modality exerts a steadfast and predictable endothelial influence on arterial tone (dotted line) that is constantly tuned by prevailing conditions.