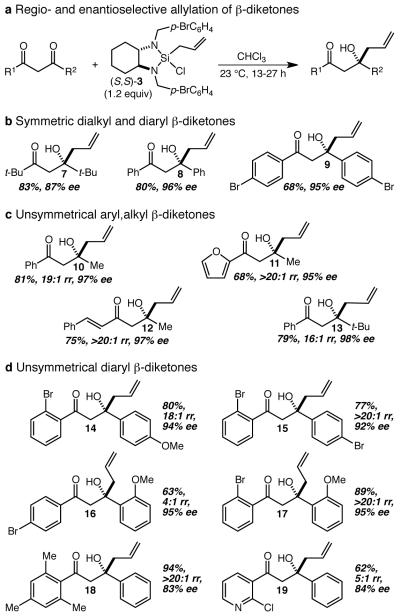
Figure 3. Scope of the regio- and enantioselective allylation of β-diketones.
a The allylation reactions were conducted by treating the β-diketone with 1.2 equiv of (S,S)-3 in CHCl3, and stirring the resultant mixture at ambient temperature for 13-27 h. In all cases the reported yield refers to the yield of isolated (≥20:1 regioisomeric purity) major product after work-up and purification by flash chromatography, and the enantiomeric excess (ee) was determined by chiral high-performance liquid chromatography. b Enantioselective allylation of symmetrical β-diketones. c Regio- and enantioselective allylation of unsymmetrical aryl,alkyl β-diketones (rr = regioisomer ratio). d Regio- and enantioselective allylation of unsymmetrical diaryl β-diketones.