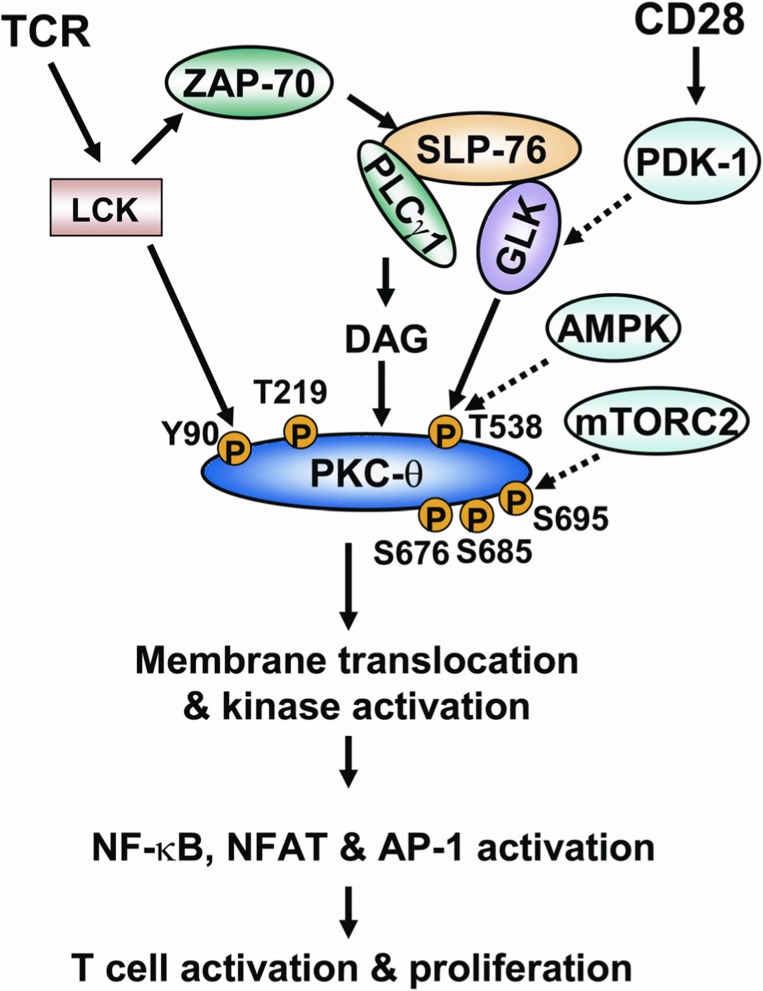
FIGURE 3.
The model of regulation of PKC-θ phosphorylation during TCR signaling. Ligation of TCR induces the activation of tyrosine kinase LCK. LCK activates ZAP-70, which in turn induces tyrosine phosphorylation of the adaptor SLP-76. SLP-76 directly interacts with and activates PLCγ1 and GLK. PLCγ1 cleaves a phospholipid, generating the second messenger diacylglycerol. The binding of diacylglycerol with PKC-θ induces the conformational change of PKC-θ; T538 of PKC-θ is then phosphorylated by GLK, leading to catalytic activation of PKC-θ. The CD28 costimulatory activates PDK-1, which facilitates PKC-θ T538 phosphorylation possibly via activating GLK. AMPK is also implicated in PKC-θ T538 phosphorylation in T cells. LCK directly interacts with and phosphorylates PKC-θ at Y90. The phosphorylation of Y90 and another residue (T219) induces membrane translocation of PKC-θ. S676, S685, and S695 residues are also phosphorylated on PKC-θ upon TCR stimulation. mTORC2 activity is required for PKC-θ S695 phosphorylation in T cells. Catalytic activation and membrane translocation of PKC-θ lead to the activation of transcription factors NF-κB, NF-AT, and AP-1, and to subsequent T cell activation.