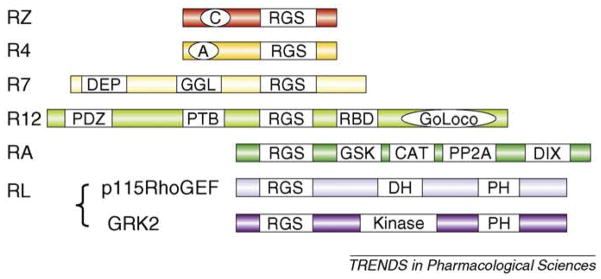
Figure I.
RGS protein subfamilies. The RZ family includes RGS17 (also known as RGSZ2), RGS20 (RGSZ1) and RGS19 (GAIP). The R4 family comprises a large number of proteins (i.e. RGS1, RGS3, RGS2, RGS4, RGS8, RGS13, RGS16 and RGS18). The RZ and R4 families are GAPs with either a poly-cysteine region (C) that is likely to be reversibly palmitoylated or an amphipathic helix (A). The N-terminal region of the R4 family seems to be important for receptor selectivity. The R7 family comprising RGS6, RGS7, RGS9 and RGS11 is more complex with protein-binding domains. The N-terminal DEP binds to R7BP and the GGL (Gγ-like) domain binds to Gβ5. The R12 family comprises RGS10, RGS12 and RGS14. RGS12 and RGS14 are larger with a RBD (Rap1/2-binding) domain and a GoLoco motif that can bind to Gα to displace Gβγ and inhibit guanine nucleotide release; RGS12 has additional N-terminal protein-binding domains (PTBs). Members of the RA family (axin and conductin) act as scaffolds binding the transcription factor β-cartenin (CAT) and controlling its phosphorylation through binding domains for glycogen synthase kinase (GSK3β) and protein phosphatase 2A (PP2A); there is also a dimerization domain (DIX). The RL subfamily is diverse and the RGS domain has low homology. Important examples include GRK2 and p115RhoGEF with a PH (pleckstrin homology) domain and either a DH (dbl homology) or kinase domain, respectively.