Table 3.
Steady-state non-compartmental pharmacokinetic parameters of linagliptin after multiple oral doses of 5 mg linagliptin in patients with mild or moderate hepatic impairment compared with healthy subjects
| Hepatic impairment group | ||||||
|---|---|---|---|---|---|---|
| Healthy (n = 8) | Mild (n = 8) | Moderate (n = 8§) | ||||
| gMean | gCV | gMean | gCV | gMean | gCV | |
| AUCτ,ss (nmol l−1 h) | 254 | 18.9 | 191 | 27.2 | 217 | 26.0 |
| Cmax,ss (nmol l−1) | 20.8 | 38.6 | 13.4 | 55.8 | 19.2 | 52.5 |
| tmax,ss (h)* | 1.50 | 0.50–2.00 | 1.00 | 0.50–3.00 | 0.625 | 0.25–2.00 |
| C24,ss (nmol l−1) | 8.41 | 18.2 | 6.75 | 28.2 | 7.85 | 18.8 |
| t1/2,ss (h) | 77.7 | 32.6 | 95.0 | 18.0 | 96.1 | 54.7 |
| fe(0,24 h),ss (%) | 7.12 | 50.3 | 4.84† | 57.8 | 6.13 | 51.2 |
| CLR,(0,24 h),ss (ml min−1) | 49.5 | 40.8 | 44.7† | 40.1 | 49.8 | 50.8 |
| RA,AUC(0,24 h) | 1.34 | 22.2 | 1.25‡ | 23.9 | 1.46 | 28.4 |
 |
1.20 | 53.9 | 1.22‡ | 64.3 | 1.53 | 65.8 |
| Accumulation t1/2 (h) | 10.9 | 66.2 | 8.11‡ | 86.8 | 13.1 | 61.7 |
For tmax, the median and range (min–max) is given.
Urine linagliptin concentrations only available for whole of day 1 for seven patients in the mild impairment group.
Single dose and steady-state pharmacokinetic data only available for seven patients in the mild impairment group.
Eight patients only completed full 7 days of study in the moderate impairment group. AUCτ,ss area under the plasma concentration–time curve at steady-state over the dosing interval τ, Cmax,ss maximum plasma concentration at steady-state, tmax,ss time from last dosing to maximum plasma concentration at steady-state over the dosing interval τ, C24,ss plasma concentration at 24 h at steady-state, t1/2,ss terminal half-life in plasma at steady-state, fe(0,24 h),ss fraction excreted unchanged in urine in the time interval 0 to 24 h at steady-state; CLR(0,24 h),ss renal clearance in the time interval 0 to 24 h at steady-state, RA,AUC(0,24 h) accumulation ratio based on AUC(0,24 h), 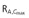 accumulation ratio based on Cmax, Accumulation t1/2 equals t× ln(2)/ln(RA,AUC(0,24 h)/[RA,AUC(0,24 h)– 1]).
accumulation ratio based on Cmax, Accumulation t1/2 equals t× ln(2)/ln(RA,AUC(0,24 h)/[RA,AUC(0,24 h)– 1]).
