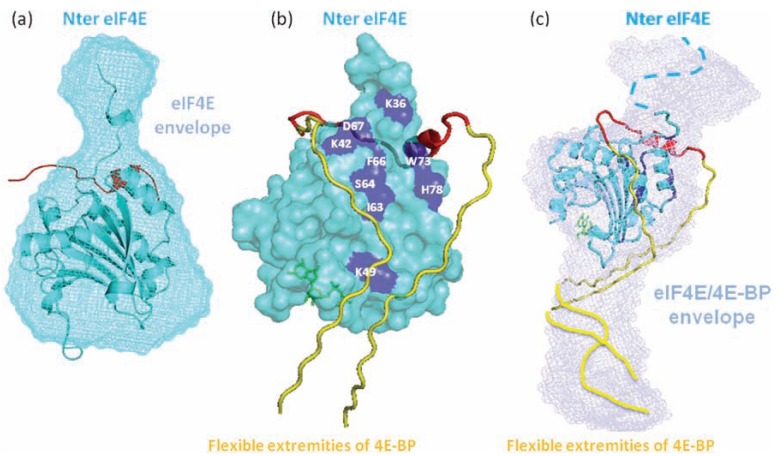
Fig. (4).
Shape calculation and 3D model of the complex eIF4E bound to 4E-BP. (a) The crystal structure of 4E-BP (cyan) is perfectly superimposable on the envelope of the free protein in solution calculated with DAMMIN from the scattering data, with a small bulge corresponding to the disordered N-terminus of 4E-BP. (b) X-ray crystallization showed that a short region of eIF4E (red) visible in the electronic density undergoes an induced folding into an alpha-helix upon binding to 4E-BP. NMR studies identified other residues of 4E-BP involved in the interaction (dark blue) on the opposite side of the protein where the alpha-helix of eIF4E binds 4E-BP, suggesting that eIF4E (yellow) wraps around 4E-BP but retains enough flexibility to not be seen by X-ray crystallography. (c) The envelope of the complex calculated with DAMMIN exhibits an upper-half region identical to the shape of 4E-BP alone and an elongated region on one side of 4E-BP, probably corresponding to the disordered portion of eIF4E. This envelope, together with data from X-ray crystallography and NMR, allowed the authors to propose a model of the complex with a rather well defined region in the close vicinity of 4E-BP and a loose region corresponding to the rest of eIF4E that remains mostly disordered in the complex (yellow). (Figure adapted from [76]).