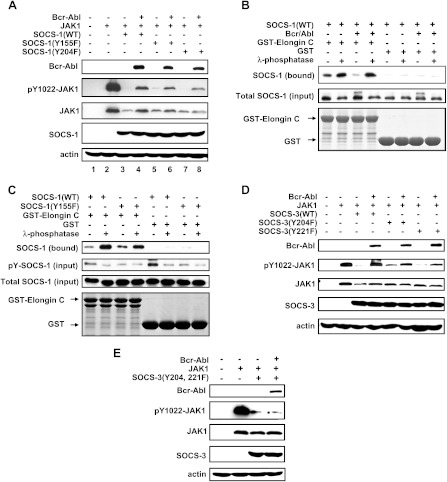
Figure 3.
Bcr-Abl-dependent phosphorylation of SOCS-1 and SOCS-3 alters their inhibitory effects on JAK1 activation and disrupts interaction between SOCS-1 and Elongin BC complex. (A) JAK1 was cotransfected with empty vector, wild-type, or mutant SOCS-1 with or without Bcr-Abl in 293T cells as described in Figure 1. The levels of protein expression and tyrosine-phosphorylated JAK1(pY-JAK1) were examined by Western blot analysis. (B) The interaction between SOCS-1 and Elongin BC was examined by in vitro binding assays. Glutathione beads coupled to either GST alone or GST-Elongin C were incubated with extracts derived from 293T cells overexpressing Bcr-Abl and SOCS-1 and either treated with λ phosphatase or mock treated. SOCS-1 bound was detected by Western blot analysis. (C) GST pull-down experiments with glutathione beads were performed as described in B. Either GST beads or GST-Elongin C beads were incubated with extracts from K562 cells ectopically expressing SOCS-1(WT) or SOCS-1(Y155F). Cell lysates were either mock or λ phosphatase treated. The immunoblots were probed as indicated. (D and E) 293T cells were cotransfected with JAK1 and either empty vector, SOCS-3(WT), SOCS-3(Y204F), and SOCS-3(Y221F) (D) or SOCS-3(Y204, 221F) (E) with or without Bcr-Abl. Protein levels and pY-JAK1 were examined by Western blot analysis using indicated antibodies.