Table 2.
Rhodium-Catalyzed Enantioselective Intramolecular Hydroamination of Aminoalkenes[a]
| entry | alkenyl amine | product | T(°C) | t(h) | yield [%][b] | ee [%][c] |
|---|---|---|---|---|---|---|
| 1 |
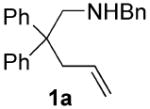
|
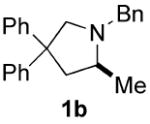
|
70 | 15 | 91 | 80(S) |
| 2 | 50 | 24 | 90 | 83(S) | ||
| 3 |
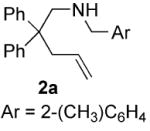
|
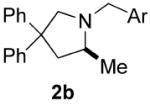
|
70 | 15 | 92 | 84(S) |
| 4d | 70 | 20 | 88 | 84(S) | ||
| 5 | 50 | 24 | 91 | 88(S) | ||
| 6 |
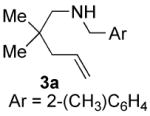
|
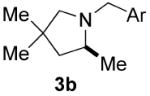
|
70 | 20 | 75 | 62(S) |
| 7 |
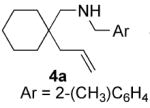
|

|
70 | 20 | 80 | 63(S) |
| 8e |
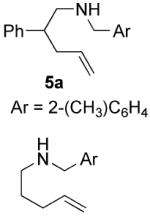
|
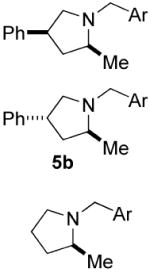
|
70 | 20 | 80 (1.1:1) | 87(2S, 4S) |
| 91(2S, 4S) | ||||||
| 9f | Ar = C6H5(6a) | 6b | 70 | 20 | 48 | 90(S) |
| 10g | Ar = 2-(CH3)C6H4(7a) | 7b | 70 | 20 | 50 | 86(S) |
| 11g | Ar = 4-(Cl)C6H4 (8a) | 8b | 70 | 20 | 63 | 85(S) |
| 12g | Ar = 4-(MeO)C6H4(9a) | 9b | 70 | 30 | 35 | 85(S) |
| 13g | Ar = 4-(CO2Me)C6H4 (10a) | 10b | 70 | 20 | 61 | 83(S) |
| 14h |

|

|
100 | 10 | 85 | 64(S) |
Reaction conditions: 0.5 mmol alkenyl amine, 5 mol% of [Rh(COD)2]BF4, 6 mol% of ligand L9, dioxane (0.5 mL).
Isolated yields (average of two runs).
The ee values were determined by chiral HPLC, GC or 1H NMR analysis of its derivatives. The absolute configuration of 1b was determined by converting it to the known (S)-MTPA amide. The configuration of 6b, 7b, 8b, 9b and 10b were determined by converting them to the known N-α-naphthyl amide. The configuration of 11b was determined by converting it to the known N-acetyl-2-methylindoline. See supporting information for details. The configurations of other amines were assigned by analogy.
2.5 mol% Rh and 3 mol% ligand L9.
Diastereomeric ratio given in parentheses.
10 mol% Rh and 12 mol% ligand L9.
5 mol% Rh and 6 mol% ligand L8.
Yield of isolated product following derivatization with acetic anhydride.
