Abstract
Background and Aims
A common response of wetland plants to flooding is the formation of aquatic adventitious roots. Observations of aquatic root growth are widespread; however, controlled studies of aquatic roots of terrestrial herbaceous species are scarce. Submergence tolerance and aquatic root growth and physiology were evaluated in two herbaceous, perennial wetland species Cotula coronopifolia and Meionectes brownii.
Methods
Plants were raised in large pots with ‘sediment’ roots in nutrient solution and then placed into individual tanks and shoots were left in air or submerged (completely or partially). The effects on growth of aquatic root removal, and of light availability to submerged plant organs, were evaluated. Responses of aquatic root porosity, chlorophyll and underwater photosynthesis, were studied.
Key Results
Both species tolerated 4 weeks of complete or partial submergence. Extensive, photosynthetically active, aquatic adventitious roots grew from submerged stems and contributed up to 90 % of the total root dry mass. When aquatic roots were pruned, completely submerged plants grew less and had lower stem and leaf chlorophyll a, as compared with controls with intact roots. Roots exposed to the lowest PAR (daily mean 4·7 ± 2·4 µmol m−2 s−1) under water contained less chlorophyll, but there was no difference in aquatic root biomass after 4 weeks, regardless of light availability in the water column (high PAR was available to all emergent shoots).
Conclusions
Both M. brownii and C. coronopifolia responded to submergence with growth of aquatic adventitious roots, which essentially replaced the existing sediment root system. These aquatic roots contained chlorophyll and were photosynthetically active. Removal of aquatic roots had negative effects on plant growth during partial and complete submergence.
Keywords: Adventitious roots, aquatic plants, aquatic roots, Cotula coronopifolia, flooding, Meionectes brownii, Haloragis brownii, root porosity, root photosynthesis, submergence tolerance, underwater photosynthesis, wetland plants
INTRODUCTION
A common response of wetland plants to flooding is the formation of an adventitious root system. These stem-borne roots can stay suspended in the water column (aquatic roots), or grow down into the sediment. Adventitious roots are adapted to the flooded environment and may support or replace the primary root system (Jackson and Drew, 1984).
As a flooding response, the growth and physiology of aquatic roots produced by woody perennials have received some attention (Hook et al., 1970; Gomes and Kozlowski, 1980; Islam and Macdonald, 2004; Iwanaga and Yamamoto, 2008; Rich et al., 2008); however, the response in herbaceous species is underrepresented in the scientific literature. Aquatic root growth as a response to flooding has been observed across a range of herbaceous crop species such as corn (Jat et al., 1973), tomato (Kramer, 1951; Jackson, 1955; Vidoz et al., 2010) and rice (Suge, 1985; Bleecker et al., 1986), as well as numerous other non-crop species (Bergman, 1920; Etherington, 1984; Javier, 1985; Osundina and Osonubi, 1989; Shimamura et al., 2007). However, while observations of aquatic root growth are commonly reported, studies of aquatic root growth and function or investigations using root removal to test the contribution of adventitious roots to flooding tolerance in herbaceous species are scarce. Previous studies of aquatic roots have all focused on partially submerged plants and used very shallow flooding regimes (10-to-50 mm). This paper describes a series of experiments investigating tolerance of partial- and complete-submergence, and aquatic root growth and physiology in two herbaceous, perennial wetland species.
In late 2007, a survey of common wetland species of south-western Australia found that many produced aquatic adventitious roots (our own unpublished observations). Cotula coronopifolia and Meionectes brownii (syn. Haloragis brownii; Moody and Les, 2007) were chosen as focus species. Both species are tolerant to extended periods of flooding during which both grow numerous stem-borne aquatic adventitious roots (our own field observations); however, the two species differ in their habitat preferences making for an interesting species comparison. Cotula coronopifolia fringes lakes and streams across Europe and continents in the southern hemisphere (Van der Toorn, 1980). This species grows primarily in drained or damp soils with extended seasonal periods of shallow, partial submergence (Patterson, 1978); there is no record of C. coronopifolia surviving full submergence, although we have observed it can survive short periods of infrequent submergence in the field. Meionectes brownii is an Australian wetland endemic, which can grow completely emergent year round on damp soils; however, it is more commonly found in areas where for large parts of the year shoots are partially or completely submerged by water up to 1 m (Marchant et al., 1987).
The first experiment evaluated biomass partitioning of plants in completely submerged, partially submerged (to one-third of shoot height), waterlogged (roots in stagnant agar, shoots in air) and aerated (roots in aerated solution, shoots in air) treatments. In this first experiment we also measured a range of physiological responses to flooding, both in shoots and roots, to gain an understanding of flooding response in the different tissues as well as whole plant responses. A second experiment was conducted in which aquatic roots were removed and growth parameters ascertained to gain insights into whether these roots benefit plant growth during submergence. Previous studies had found a negative response of plant growth to the removal of aquatic roots (Jackson, 1955; Javier, 1985; Tsukahara and Kozlowski, 1985; Osundina and Osonubi, 1989); however, these experiments used shallow flooding and the biomass of removed roots was small. It was therefore hypothesized that the response to aquatic root removal would be greater in more deeply or completely submerged wetland plants. A third experiment examined the effect of light on aquatic root growth and development. Light has been demonstrated to inhibit both cell elongation and division in roots of numerous species (Feldman, 1984). Aquatic roots grow into an environment that is often well illuminated, so we hypothesized that, unlike many sediment roots, growth of aquatic roots would likely be insensitive to light, as their natural environment is typically lit. Growth into an illuminated environment also results in chlorophyll formation in some aquatic roots (Rich et al., 2008; Rich et al., 2011) and we hypothesized that roots growing in lower light levels would contain less chlorophyll than those grown in higher light.
MATERIALS AND METHODS
Experimental designs
This paper describes the results of three experiments, all using plants grown in nutrient solution (described below in ‘Plant material’). All experiments were conducted on both Cotula coronopifolia L. and Meionectes brownii Hook. f. (syn. Haloragis brownii), using three replicate plants for each treatment and all used the same submergence set-up (described below).
Experiment 1 was designed to examine growth and response of tissue net photosynthesis, soluble sugars and chlorophyll concentrations to different depths of flooding. The treatments used were: (a) a control, emulating completely emergent plants, with shoots in air and sediment roots in an aerated nutrient solution; (b) simulated soil waterlogging by placement of sediment roots in stagnant liquid agar nutrient solution (see ‘Plant materials’ below), with shoots in air; (c) shoots partially submerged (water depth was one-third shoot height) and the sediment roots were in stagnant liquid agar nutrient solution; (d) shoots fully submerged and sediment roots in stagnant liquid agar nutrient solution. Plants underwent treatment for 28 d and both floodwater and sediment root stagnant 0·1 % agar nutrient solutions were renewed weekly. Initial and final harvests were carried out to quantify fresh and dry masses of submerged and aerial stems and leaves, aquatic and sediment roots (oven dried at 60 °C for 72 h). During harvest, fresh tissues were used to determine porosity and underwater net photosynthetic rates (both described below) and subsamples of all tissues were wrapped in aluminium foil, frozen in liquid N2 and freeze-dried for soluble sugar and chlorophyll analyses (described below).
Experiment 2 was designed to investigate the relationship between aquatic root biomass and plant growth during flooding. This experiment had a 2 × 3 factorial design, utilizing two flooding depths (partial and complete shoot submergence, with sediment roots in stagnant 0·1 % agar nutrient solution) and three aquatic root removal regimes, every second day, after 14 d and no removal (control). Plants were submerged for 28 d and both floodwater and sediment root stagnant 0·1 % agar nutrient solutions were renewed weekly. All plants were completely removed from the submergence solution for 15 min every second day, regardless of treatment, and during this time aquatic roots were removed as required for the various treatments. Initial and final harvests were carried out to quantify fresh and dry masses of submerged and aerial stems and leaves, aquatic and sediment roots (oven dried at 60 °C for 72 h). All removed aquatic root tissue was also oven dried and weighed.
Experiment 3 examined the effect of light availability on aquatic root growth, porosity, chlorophyll formation and underwater net photosynthetic rates. This experiment utilized partially submerged plants (with sediment roots in stagnant 0·1 % agar nutrient solution) under three low-light treatments in the submergence solution, and a non-shaded control. Plants were grown in a sunlit 20/15 °C day/night phytotron, and light availability within individual submergence tanks was manipulated through the use of neutral density filters (Lee Filters, Burbank, CA, USA). Filters were wrapped around the tanks to 1 cm above the submergence depth and also floated on the water surface with a 1-cm-diameter hole cut for the stem to emerge. Photosynthetically active radiation (PAR) was logged for 24 h in each tank using an underwater sensor connected to a data logger (LI-192 sensor and LI-1400 data logger; Licor Biosciences, Lincoln, NE, USA). The three treatments received PAR daily means (μmol m−2 s−1 ± s.e.) and maximums (maximum was recorded for as little as 5 min near midday; μmol m−2 s−1) of 4·7 ± 2·4 (maximum of 20), 22·7 ± 1·7 (maximum of 60), 48·3 ± 1·9 (maximum of 100), with the non-shaded (sunlit) control receiving 616·4 ± 17·2 (maximum of 1200). Plants underwent treatment for 28 d, with floodwater and sediment root stagnant 0·1 % agar nutrient solutions renewed at 14 d. Initial and final harvests were carried out to quantify fresh and dry masses of submerged and aerial stems and leaves, aquatic and sediment roots (oven dried at 60 °C for 72 h). During harvest, fresh tissues were used to determine porosity and underwater net photosynthetic rates (described below), and subsamples of all tissues were wrapped in aluminium foil, frozen in liquid N2 and freeze-dried for chlorophyll analyses (described below).
Plant material
Cotula coronopifolia was collected from Herdsman Lake in Perth, Western Australia (31·92738°S, 115·809588°E) and M. brownii from an unnamed ephemeral wetland near Albany, Western Australia (35·0779°S, 117·9267°E). Shoot cuttings (100–150 mm) were collected, wrapped individually in paper towel dampened with wetland water, placed in plastic bags within an insulated cool-box, and transported on the same day to a 20/15 °C day/night phytotron at The University of Western Australia.
Axial shoots (30 mm long) were propagated hydroponically in an aerated nutrient solution, initially at 25 % concentration and increased by 25 % weekly until full strength was reached. Full-strength nutrient solution contained (in mol m−3): NO3− 3·38, K+ 3·00; SO42– 1·43, Ca2+ 1·13, NH4+ 0·47, Mg2+ 0·30, HPO42– 0·15, Na+ 7·58·10−2, H4SiO4− 7·50·10−2, Cl− 3·75·10−2, Fe-EDTA 3·75·10−2, H3BO3 1·88·10−2, Mn2+ 1·50·10−3, Zn2+ 1·50·10−3, Ni2+ 7·50·10−4, Cu2+ 3·75·10−4, Mo2+ 3·75·10−4, and 1·87 MES, with the pH adjusted to 6·5 with KOH, in deionized water. Plants not used as aerated controls in expt 1 were pretreated for 24 h on hypoxic nutrient solution (nutrient solution bubbled with N2) and 1 week in stagnant agar nutrient solution [nutrient solution of the same composition as above, containing 0·1 % (w/v) agar, bubbled overnight with N2 to remove O2 prior to use]. Roots of these plants were then placed in 2·25-L black plastic bottles of fresh deoxygenated 0·1 % agar nutrient solution and the bottles sealed with a foam plug holding the stem 1 cm above the roots. Plants were left overnight and submergence treatments were imposed the next day. In expt 1, aerated control plants received no pretreatment and were placed in 2·25-L black plastic bottles containing fresh, aerated, full-strength nutrient solution. These plants had air continuously bubbled into the nutrient solution via thin tubes. The size constraint of the submergence system (see below) limited experiments to three replicate plants per treatment (each plant in an individual tank); therefore, numerous plants were grown but only the most homologous were used in experiments (e.g. in expt 1 the mean total plant dry mass at the time treatments were imposed for C. coronopifolia was 6·1 ± 0·1 g and of M. brownii was 6·6 ± 0·5 g). Treatments were imposed on plants 10 weeks after initial propagation.
Submergence tank experimental set-up
Individual plants in 2·25-L black plastic bottles were placed into individual cylindrical Perspex tanks (diameter 20 cm, height 50 cm) and, depending on treatment, shoots were left in air or submerged (fully or partially) in a submergence solution containing (in mol m−3): Ca2+ 0·50; Mg2+ 0·25; Cl− 1·00; SO42− 0·25; K+ 1·00, in deionized water. To regulate CO2 availability, the solution also contained 1·0 mol m−3 KHCO3 and the free CO2 concentration was maintained at 200 mmol m−3 (representing the mean daily free CO2 concentration found at the Albany collection site; data not shown) by adding pressurized CO2 regulated by a pH controller (α-control; Dupla Aquaristik, Bielefeld, Germany). Submergence tanks were connected to a filtering system with ultraviolet light sterilization and physical filters (Fishmate 1000PS, Pet-Mate, Arlington, TX, USA) that minimized algal growth and ensured circulation of the submergence water. Solution that evaporated was replaced with deionized water. All experiments were conducted in a sunlit 20/15 °C day/night phytotron, maximum PAR in expts 1 and 2 was 1200 µmol m−2 s−1.
Underwater net photosynthesis (PN)
Underwater net photosynthesis (PN) of plant tissues was measured as net O2 evolution in sealed glass bottles mounted on a rotating wheel incubator (Colmer and Pedersen, 2008). The glass bottles (50 mL) contained submergence solution (see above). Measurements were started at approx. 50 % of air equilibrium for O2 (solution pre-bubbled with N2 and air in 1 : 1 volumes), with KHCO3 injected into the mixed solution just prior to filling the bottles to achieve 500 mmol m−3 of available CO2 at pH 6 (Stumm and Morgan, 1996). This CO2 concentration was chosen as it is close to the level needed for CO2-saturated underwater PN (Rich et al., 2011) and matched the highest concentrations measured during the morning at the Albany collection site (data not shown). Tissue (two or three leaves or eight 50-mm root segments or three 30-mm stem segments) was placed into bottles with two glass beads (2 mm diameter) to facilitate mixing. The bottles were immediately mounted in the incubator, with bottles without tissues serving as blanks. Following incubations of approx. 1·5 h, dissolved O2 concentrations in the solutions were measured using a mini-electrode with protection cap (OX-500; Unisense A/S, Aarhus, Denmark) connected to a picoammeter (PA2000; Unisense A/S). Measurements were conducted at 20 °C with PAR of 430 ± 7 µmol m−2 s−1. The projected area of leaves was measured using a leaf-area meter (Li-Cor LI-3000; Lincoln, NE, USA). Stem and aquatic root diameters were measured using digital callipers and their surface areas were calculated using the formula for a cylinder.
Tissue characterization: porosity, chlorophyll and soluble sugars
Porosity (% gas volume per unit tissue volume) of sediment and aquatic roots was measured by determining tissue buoyancy before and after vacuum infiltration of the gas spaces with water (Raskin, 1983), using the equations as modified by Thomson et al. (1990).
Total chlorophyll was extracted from freeze-dried and ground tissues (10–20 mg dry mass) in cold, 100 % methanol (1·25 mL) for 30 min, in darkness (Wellburn, 1994). After centrifugation at 9300 g for 10 min at 4 °C, supernatants were collected for analysis. Concentrations of chlorophylls a (chla) and b (chlb) were determined by measuring absorbance of the samples at 665·2 and 652·4 nm, using a glass cuvette in a UV-visible spectrophotometer (model 1601; Shimadzu, Tokyo, Japan), and the equations from Wellburn (1994).
Total soluble sugars were measured from tissues collected and frozen in liquid N2 at dusk. Tissues were freeze-dried and ground in a ball mill prior to extraction. Sugars were extracted from 10–20 mg dry mass in 1 mL of 80 % ethanol : 20 % deionized water. This suspension was boiled under reflux for 20 min, twice. Total sugars were measured colourmetrically using anthrone (Yemm and Willis, 1954). Total sugar concentrations (as hexose equivalents) were determined by measuring the absorbance of the samples at 620 nm using a glass cuvette in a UV-visible spectrophotometer (model 1601; Shimadzu), and relating these values to a standard curve for glucose. Soluble sugar recovery was determined to be 85 % using glucose spiked into additional tissue samples immediately prior to extraction (data presented here not adjusted).
Data analyses
Statistical analyses (Student's t-tests, ANOVA and Fishers LSD) were undertaken using GenStat 10·0 (VSN International, Hemel Hempstead, UK). Relative growth rates (RGR) were determined as (ln of final dry mass – ln of initial dry mass)/time.
RESULTS
Plant growth under different flooding regimes
Meionectes brownii and C. coronopifolia grew extensive aquatic root systems in response to flooding (Fig. 1). Both species produced aquatic roots from stem nodes (Fig. 1C, D) and up to six roots were observed emerging from a single node, although typically there were two or three aquatic roots per node. In response to partial and full submergence, plants of both species produced >100 new aquatic roots. Partial submergence of M. brownii and C. coronopifolia resulted in aquatic roots approx. 2-fold longer than when plants were fully submerged; however, the diameter and porosity of aquatic roots from both species were not significantly different under the two submergence treatments (Tables 1 and 2). Porosity of sediment roots could not be determined for most submerged treatments as these roots were flaccid and even partially decayed; the exception being fully submerged M. brownii which had healthy-looking sediment roots and produced new sediment adventitious roots with porosity of 35·5 ± 1·4 %. In both species, the porosity of aquatic roots was significantly higher (P ≤ 0·05) than sediment root porosity of aerated controls. Sediment roots from the stagnant treatment had a similar high porosity to the aquatic roots in C. coronopifolia (approx. 40 %; Table 2). In contrast, the aquatic roots of M. brownii had porosity close to 20 % while porosity of sediment roots of stagnant plants of this species was 1·5-fold higher (Table 2).
Fig. 1.
Aquatic adventitious roots in Cotula coronopifolia and Meionectes brownii after partial submergence for 28 d. Cotula coronopifolia (A) and M. brownii (B) submerged to the level indicated by the white line in individual tanks in a 20/15 °C day/night phytotron grew extensive aquatic roots (open arrows). Sediment roots (closed arrows) were in stagnant deoxygenated 0·1 % agar nutrient solution. Numerous aquatic roots emerged from stem nodes in C. coronopifolia (C) and M. brownii (D). Scale bars = 5 cm
Table 1.
Characteristics of adventitious aquatic roots formed on partially and completely submerged Cotula coronopifolia and Meionectes brownii
| Species | Treatment | Length of longest aquatic roots (mm) | Diameter (mm) | Soluble sugars (dusk) (μmol hex. eq. g−1 DM) |
|---|---|---|---|---|
| C. coronopifolia | Partial submergence | 485 ± 4·0a | 1·3 ± 0·06a | 105·8 ± 3·18a |
| C. coronopifolia | Complete submergence | 255 ± 16·5b | 1·2 ± 0·03a | 13·6 ± 0·14b |
| M. brownii | Partial submergence | 327 ± 14·8a | 1·1 ± 0·08a | 56·2 ± 2·03a |
| M. brownii | Complete submergence | 186 ± 16·9b | 1·1 ± 0·08a | 20·1 ± 0·19b |
Roots were taken from plants which had been submerged in a 20/15 °C day/night phytotron for 28 d. Length and diameter were taken from the five longest roots on each replicate plant, with diameter being measured 5 cm from the root/shoot junction. Tissues for sugars were collected at sunset and are expressed in hexose equivalents (hex. eq.) on a dry mass (DM) basis. Data presented are means ± s.e., n = 3. Different letters indicate significant differences at P < 0·05 (comparisons within species for each characteristic).
Table 2.
Porosity of sediment and aquatic roots of Cotula coronopifolia and Meionectes brownii
| Porosity (% gas volume per unit root volume) |
|||
|---|---|---|---|
| Treatment | Root type | C. coronopifolia | M. brownii |
| Initial harvest | Sediment | 12·5 ± 3·0a | 15·3 ± 0·8a |
| Aerated | Sediment | 13·0 ± 1·1a | 15·8 ± 1·3a |
| Stagnant | Sediment | 38·2 ± 1·7b | 30·5 ± 1·3b |
| Partial submergence | Sediment | n.d. | n.d. |
| Complete submergence | Sediment | n.d. | 35·5 ± 1·4b |
| Partial submergence | Aquatic | 42·5 ± 2·0b | 20·9 ± 0·2c |
| Complete submergence | Aquatic | 38·2 ± 1·2b | 20·8 ± 1·9c |
Porosity was measured on approx. 0·5 g of fresh root segments taken 4–9 cm from the root/shoot junction. Data presented are means ± s.e., n = 3. Diffrent letters indicate significant differences at P < 0·05 (comparisons within species, i.e. down columns).
n.d.: not determined as these roots were too flaccid or partially decayed.
All three treatments significantly reduced whole-plant RGR in both species, except for partially submerged C. coronopifolia, which showed higher RGR compared with its aerated control (Table 3). Simulated waterlogging of ‘sediment’ roots, by use of the stagnant agar nutrient solution in the root container but with shoots remaining in air, reduced growth of both species (Table 3). So, in comparison with the aerated controls, most plants in the shoot submergence treatments also grew less (roots also in stagnant agar), but plants exposed to partial shoot submergence grew better than those with roots in stagnant agar and with the shoot in air (Table 3). Aquatic roots contributed a significant amount of the total plant dry mass in both completely and partially submerged plants (up to approx. 26 % in C. coronopifolia and approx. 24 % in M. brownii; Table 3). Aquatic root dry mass was highest in partially submerged plants, contributing over 90 % of the root dry mass (Table 3). Sediment root dry mass in both species was reduced significantly with flooding, although root to shoot ratios (R : S) increased dramatically upon submergence due to growth of aquatic roots (Table 3).
Table 3.
Total plant and tissue component dry mass, whole-plant relative growth rate (RGR) and root to shoot ratio (R : S) of Cotula coronopifolia and Meionectes brownii under different flooding regimes
| Species | Treatment | Total plant dry mass (g) | Shoot dry mass (g) | Sediment root dry mass (g) | Aquatic root dry mass (g) | Whole-plant RGR (g g−1 d−1) | R : S |
|---|---|---|---|---|---|---|---|
| C. coronopifolia | Aerated | 17·6 ± 1·7a | 16·7 ± 1·7a | 0·88 ± 0·02a | n.a. | 0·033 ± 0·001a | 0·06 ± 0·00a |
| C. coronopifolia | Stagnant | 12·6 ± 0·7b | 11·7 ± 0·42b | 0·43 ± 0·05b | n.a. | 0·0082 ± 0·007b | 0·04 ± 0·01b |
| C. coronopifolia | Partial submergence | 17·7 ± 0·6a | 12·8 ± 0·60b | 0·26 ± 0·02c | 4·6 ± 0·38a | 0·049 ± 0·002c | 0·38 ± 0·04c |
| C. coronopifolia | Complete submergence | 5·8 ± 0·4c | 4·7 ± 0·61c | 0·18 ± 0·02d | 0·98 ± 0·16b | 0·027 ± 0·002d | 0·31 ± 0·04c |
| M. brownii | Aerated | 24·9 ± 2·2a | 21·3 ± 1·8a | 3·6 ± 0·58a | n.a. | 0·049 ± 0·006a | 0·17 ± 0·02a |
| M. brownii | Stagnant | 8·9 ± 3·4b | 12·8 ± 3·5b | 0·59 ± 0·02b | n.a. | 0·023 ± 0·005b | 0·04 ± 0·01b |
| M. brownii | Partial submergence | 13·9 ± 1·8c | 12·3 ± 1·3b | 0·24 ± 0·02c | 3·4 ± 0·52a | 0·029 ± 0·005b | 0·29 ± 0·02c |
| M. brownii | Complete submergence | 7·8 ± 1·8b | 6·5 ± 0·54c | 0·45 ± 0·07d | 0·87 ± 0·06b | 0·026 ± 0·005b | 0·21 ± 0·03c |
Ten-week-old plants were completely submerged (sediment roots in stagnant deoxygenated nutrient agar solution), partially submerged to one-third of the shoot height (sediment roots in stagnant deoxygenated nutrient agar solution), or with sediment roots in stagnant deoxygenated nutrient agar solution, shoots in air or with sediment roots in aerated nutrient solution and shoots in air for 28 d. At 10 weeks, when treatment was imposed, mean total plant dry mass of C. coronopifolia was 6·1 ± 0·1 g and M. brownii was 6·6 ± 0·5 g. Data presented are means ± s.e., n = 3. Different letters indicate significant differences at P < 0·05 (comparisons down columns, within species).
n.a.: not applicable (these plants had no aquatic roots).
Photosynthesis and chlorophyll formation under different flooding regimes
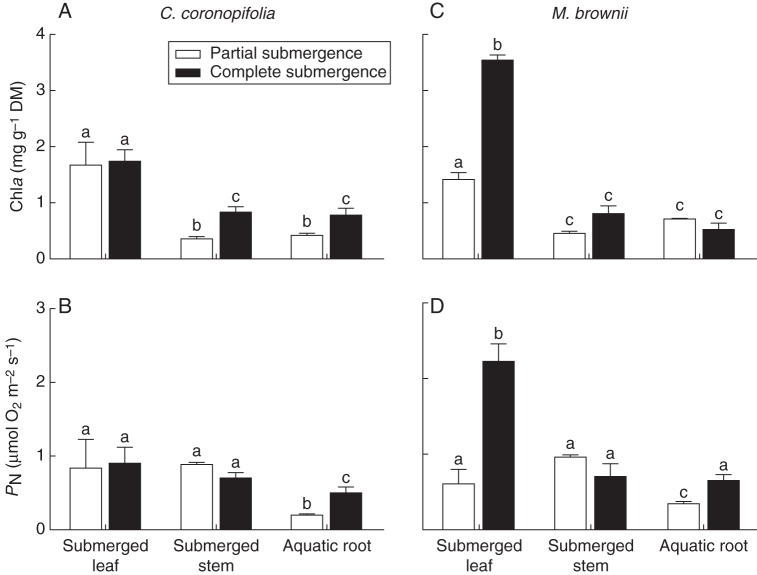
Meionectes brownii and C. coronopifolia aquatic roots contained chlorophyll and were photosynthetically active (Fig. 2). Aquatic root chla concentrations were similar to those of submerged stems; however, in both species this was significantly lower than concentrations found in submerged leaves (Fig. 2A, C). Submergence resulted in leaf chlorosis in both species. In C. coronopifolia, waterlogging or partial submergence did not affect the aerial leaves, which contained chla at 4·7 ± 0·5 mg g−1 dry mass (DM), 2·8-fold higher than the concentration found in submerged leaves of partially or completely submerged plants (Fig. 2). In M. brownii, however, flooding treatments affected not only submerged leaves but aerial leaves as well. Chla concentrations in aerial leaves of stagnant and partially submerged M. brownii were only 43 % of those in aerial leaves of the aerated controls (2·2 ± 0·1 mg g−1 DM and 5·1 ± 0·4 mg g−1 DM, respectively), a concentration not much higher than that found in submerged leaves of partially submerged plants (1·4 ± 0·2 mg g−1 DM; Fig. 2). In contrast to both the aerial and submerged leaves in other treatments, the leaves of completely submerged M. brownii showed a modest reduction in chla concentration (3·6 ± 0·08 mg g−1 DM; Fig. 2).
Fig. 2.
Chlorophyll a (Chla) and underwater net photosynthetic rates (PN) in organs of Cotula coronopifolia and Meionectes brownii grown under either partial or complete submergence for 28 d. Chla was extracted in methanol from organs of C. coronopifolia (A) and M. brownii (C); for comparison, the chla of C. coronopifolia aerial leaves in all three treatments with aerial tissues was 4·7 ± 0·5 mg g−1 DM, while that in aerial leaves of M. brownii was 5·1 ± 0·4 mg g−1 DM in the aerated treatment and 2·2 ± 0·1 mg g−1 DM in stagnant and partially submerged treatments. PN of C. coronopifolia (B) and M. brownii (D) organs was measured as O2 production in closed vials at 20 °C with 500 mmol m−3 dissolved CO2 and PAR of 430 ± 7 µmol m−2 s−1. Data presented are means ± s.e., n = 3.
Underwater net photosynthetic rates (PN) were similar for submerged stems and leaves of partially and completely submerged M. brownii and C. coronopifolia (Fig. 2B, D), with the exception of submerged leaves of completely submerged M. brownii, which far out-performed all other submerged tissues (2·2 ± 0·2 µmol O2 m−2 s−1). Aquatic root PN rates in both species were significantly lower than those of submerged leaves on a surface area basis, with the exception of aquatic roots from completely submerged M. brownii. In both species, the aquatic roots of partially submerged plants photosynthesized at lower rates than aquatic roots of fully submerged plants (Fig. 2). Regardless of higher photosynthetic rates in aquatic roots of fully submerged plants, at dusk these roots had significantly lower levels of soluble sugars (Table 1). This was especially noticeable in C. coronopifolia with the aquatic roots of fully submerged plants having 87 % lower soluble sugars than the aquatic roots of partially submerged plants.
Plant response to aquatic root removal
To examine the importance of aquatic roots to flooded plant growth, an experiment where aquatic roots were removed at different time points from both fully and partially submerged plants was conducted. As expected from expt 1 (Tables 1 and 2), partially submerged plants grew longer aquatic roots, as compared with completely submerged plants, and porosity and diameters did not differ between the treatments (data not shown).
In both species and both submergence treatments, root removal resulted in a reduction in whole-plant RGR (with aquatic root mass excluded; Table 4). With the aquatic root mass excluded, completely submerged C. coronopifolia showed apparent negative growth rates, due to decay of leaves from submerged portion of stems. With the exception of partially submerged C. coronopifolia, no significant difference was demonstrated between whole-plant RGR between the two root-removal treatments. Root removal once at 14 d in either submergence treatment did not affect the total dry mass of aquatic roots grown over 28 d (Table 4); however, removal of roots every second day resulted in significantly lower total aquatic root dry mass as root elongation was prevented. In completely submerged plants, aquatic root removal resulted in lower chla concentrations within submerged leaves; however, in partially submerged plants no change in submerged leaf chla was found in either species regardless of root removal (Table 4). Stem chla concentrations tended to decline in plants with aquatic roots removed, although the reduction was significant only in the stems of completely submerged plants (Table 4). In both species, completely submerged plants maintained higher concentrations of chla in both submerged stems and leaves than partially submerged plants.
Table 4.
Response of Cotula coronopifolia and Meionectes brownii to aquatic adventitious roots removal at different time intervals (never, once after 14 d, every second day) while under partial and complete submergence
| Species | Submergence treatment | Aquatic root removal | RGR (aquatic root mass excluded) (g g−1 d−1)* | Chla in submerged leaves (mg g−1 DM) | Chla in submerged stems (mg g−1 DM) | DM of aquatic roots removed (g DM) | Total DM aquatic roots grown (g DM) |
|---|---|---|---|---|---|---|---|
| C. coronopifolia | Partial | Never | 0·017 ± 0·001a | 1·01 ± 0·78a | 0·29 ± 0·02a | n.a. | 2·539 ± 0·224a |
| C. coronopifolia | Partial | Once on day 14 | 0·008 ± 0·003b | 1·42 ± 0·02a | 0·23 ± 0·03a | 1·196 ± 0·095a | 1·992 ± 0·248a |
| C. coronopifolia | Partial | Every 2nd day | 0·001 ± 0·001c | 1·23 ± 0·65a | 0·25 ± 0·05a | 0·228 ± 0·075b | 0·243 ± 0·078b |
| C. coronopifolia | Complete | Never | 0·005 ± 0·003b | 2·68 ± 0·14b | 0·91 ± 0·05b | n.a. | 0·639 ± 0·204c |
| C. coronopifolia | Complete | Once on day 14 | –0·005 ± 0·003c | 2·14 ± 0·19c | 0·62 ± 0·05c | 0·238 ± 0·018b | 0·490 ± 0·033c |
| C. coronopifolia | Complete | Every 2nd day | –0·007 ± 0·005c | 1·88 ± 0·16c | 0·52 ± 0·01c | 0·201 ± 0·046b | 0·219 ± 0·046b |
| M. brownii | Partial | Never | 0·014 ± 0·001a | 0·89 ± 0·22a | 0·39 ± 0·04a | n.a. | 1·481 ± 0·745a |
| M. brownii | Partial | Once on day 14 | 0·009 ± 0·002b | 0·60 ± 0·12a | 0·30 ± 0·02a | 0·999 ± 0·188a | 1·874 ± 0·625a |
| M. brownii | Partial | Every 2nd day | 0·007 ± 0·005b | 0·96 ± 0·15a | 0·33 ± 0·09a | 0·340 ± 0·240b | 0·404 ± 0·301b |
| M. brownii | Complete | Never | 0·022 ± 0·001c | 3·73 ± 0·23b | 1·89 ± 0·17b | n.a. | 1·034 ± 0·456c |
| M. brownii | Complete | Once on day 14 | 0·016 ± 0·003ac | 2·89 ± 0·08c | 0·9 ± 0·13c | 0·348 ± 0·078b | 1·036 ± 0·502c |
| M. brownii | Complete | Every 2nd day | 0·018 ± 0·004ac | 2·44 ± 0·10c | 0·51 ± 0·09d | 0·115 ± 0·006c | 0·173 ± 0·047d |
Relative growth rate (whole-plant excluding aquatic roots; RGR), chlorophyll a concentration (chla), aquatic root dry mass (DM) of total removed and grown (mass at final harvest plus removed mass) are shown for the 28-d treatment period. To allow for comparisons within the table, the mass of aquatic roots was not included within the RGR. When aquatic root masses were included in the RGR of the controls (no aquatic root removal) whole-plant RGR did not differ significantly (P < 0·05) from those in expt 1 (Table 4). Data presented are means ± s.e., n = 3. Different letters indicate significant differences at P < 0·05 (comparisons down columns, within species).
n.a.: not applicable (these plants had no aquatic roots removed).
* Sediment roots included, aquatic roots removed from calculation.
Aquatic root development and chlorophyll formation under different light availabilities
In expt 3, in which partially submerged plants with varying PAR available to submerged tissues were used, no significant difference in aquatic root dry mass accumulation was found over the 28-d experiment, regardless of light availability; C. coronopifolia gained 0·06 ± 0·01 g DM d−1 of aquatic roots while M. brownii gained 0·14 ± 0·01 g DM d−1. The morphology of these aquatic roots also was not affected by PAR level, and their length and diameter did not differ from those seen in partially submerged plants in the initial growth experiment (data not shown for this experiment; expt 1 root lengths and diameters in Table 1).
Experiment 3 illustrated that only low levels of PAR are needed for chlorophyll synthesis within aquatic roots; in both M. brownii and C. coronopifolia, chla and chlb were detected when roots had been exposed to a daily mean PAR of only 4·7 ± 2·4 µmol m−2 s−1 (short maximum PAR of 20 µmol m−2 s−1; Fig. 3). Concentrations of chla in both species reached levels not significantly different to the control (full sunlight) plants when aquatic roots were exposed to daily mean PAR of 22·7 ± 1·7 µmol m−2 s−1 (short maximum of 60 µmol m−2 s−1; Fig. 3). In both species, chlb showed little change in concentration regardless of light treatment (Fig. 3). In both species, aquatic root underwater PN rates, measured at PAR of approx. 430 µmol m−2 s−1, initially increased with higher PAR during growth (up to 48·3 ± 1·9 µmol m−2 s−1; short maximum of 100 µmol m−2 s−1), above this light intensity there was no significant further increase in PN.
Fig. 3.
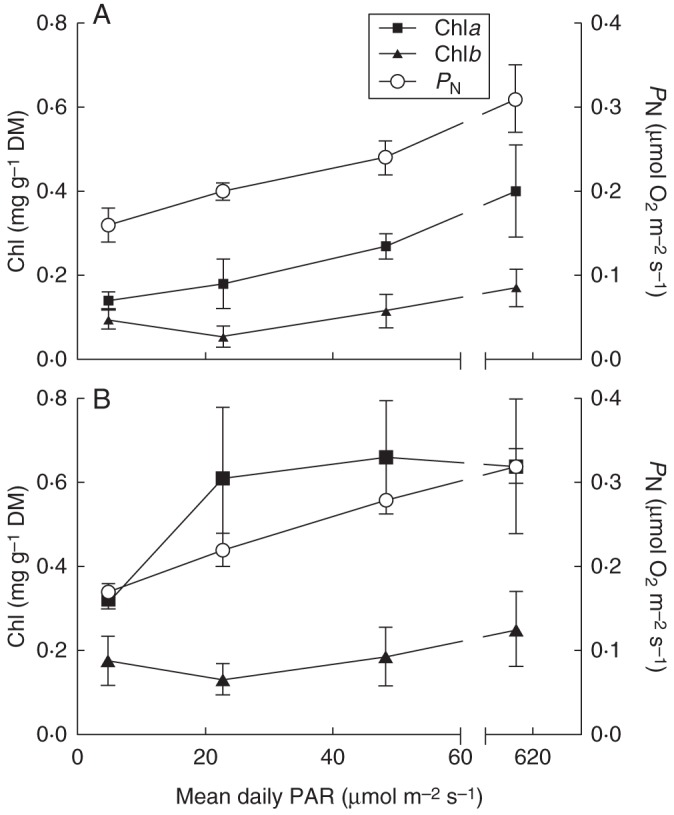
Underwater net photosynthetic rates (PN) and chlorophyll concentration (chla and chlb) in aquatic roots of Cotula coronopifolia (A) and Meionectes brownii (B) grown with different levels of light (PAR) available to submerged tissues. The three treatments received PAR daily means (μmol m−2 s−1) of 4·7 ± 2·4 (maximum of 20), 22·7 ± 1·7 (maximum of 60), 48·3 ± 1·9 (maximum of 100) with the non-shaded control plants receiving 616·4 ± 17·2 (maximum of 1200). PN was measured as O2 production in closed vials at 20 °C with 500 mmol m−3 dissolved CO2 and PAR of 430 ± 7 µmol m−2 s−1. Data presented are means ± s.e., n = 3.
DISCUSSION
Both C. coronopifolia and M. brownii tolerated 4 weeks of complete submergence (the duration of the present experiments). Simulated waterlogging of ‘sediment’ roots, by use of the stagnant agar nutrient solution, reduced growth of both species. So, in comparison with the aerated controls, most plants in the shoot submergence treatments also grew less (roots also in stagnant agar), with the exception of partially submerged C. coronopifolia. Both species responded to complete and partial submergence by growth of an extensive aquatic adventitious root system from submerged stems. These new aquatic roots were a major constituent of the total plant dry mass (approx. 26 % in C. coronopifolia and approx. 24 % in M. brownii; Table 3) and constituted over 90 % of the total root dry mass in partially submerged plants. For both species, plants exposed to partial shoot submergence grew better than those with roots in stagnant agar and with the shoot in air (Table 3); we therefore hypothesize that production of adventitious aquatic roots to replace the severely impeded ‘sediment’ roots in stagnant agar might have contributed to this improved growth (tested by the root removal experiment; discussed below). Aquatic root biomass was not affected by light availability to submerged shoots with emergent shoots non-shaded, although aquatic root chlorophyll concentrations were lower at low PAR. These new aquatic roots potentially confer some benefit(s) to both species during flooding, as aquatic root pruning resulted in reduced whole-plant RGR in both completely and partially submerged plants.
The responses of root systems to inundation are highly variable; however, due to a combination of reduced primary root growth in anoxic sediments (Webb and Armstrong, 1983) and root death (Kozlowski, 1984; Vartapetian and Jackson, 1997), there is generally a reduction in the root to shoot (R : S) ratio for plants in waterlogged sediments (Kozlowski, 1984; Pezeshki, 2001). Large declines in the R : S ratio can occur even in flood-tolerant wetland species such as Rumex palustris, which showed a 50 % reduction when waterlogged, while the ratio in the less tolerant Rumex crispus was reduced by 70 % (Voesenek et al., 1989), despite production of sediment adventitious roots by both these species (Visser et al., 1996). Even flooded paddy rice has a relatively low R : S ratio of 0·13–0·23 depending on variety and age (Teo et al., 1995), as compared with 0·4–0·55 in dry-land cereals such as wheat when in drained soil (Siddique et al., 1990). Both species studied here followed this trend with extremely low sediment R : S ratios in waterlogged (stagnant treatment) plants (approx. 0·04 in both species; Table 3). In submerged plants of both species, however, the growth of large aquatic root systems resulted in higher R : S ratios compared with aerated, emergent controls; being up to 6·3-fold (C. coronopifolia) and 1·7-fold (M. brownii) higher (Table 3).
Most studies on aquatic adventitious roots have been conducted under shallow flooding depths (≤50 mm); consequentially, only small sections of the stem were submerged and able to produce aquatic roots (see Introduction for references). Even though the aquatic root biomass produced by plants in these studies was relatively low, aquatic root removal was usually detrimental to plant growth. Tsukahara and Kozlowski (1985) found removing aquatic roots from Platanus occidentalis seedlings significantly reduced stem height and diameter, and reduced RGR of leaves and roots by approx. 25 %. Biomass accumulation was reduced and leaf abscission increased through aquatic root removal in several herbaceous species (Javier, 1985; Osundina and Osonubi, 1989), and in tomato aquatic root removal resulted in lower growth rates; however, leaf abscission was not affected (Jackson, 1955). In the present study, aquatic root removal was detrimental to the RGR of both species, although RGR only differed with various frequencies of root removal in partially submerged C. coronopifolia. Within a submergence treatment, submerged leaf chla concentration was not affected in either C. coronopifolia or M. brownii, although in completely submerged plants, aquatic root removal every second day significantly reduced submerged stem chla concentration (Table 4). The apparent negative impact of removing aquatic roots during flooding, both for partially and completely submerged plants, indicates some benefit may be conferred by these roots to plant health and biomass accumulation during flooding.
It is common for flood-tolerant species to grow an adventitious root system when inundated (Jackson and Drew, 1984; Colmer and Voesenek, 2009). In the flood-tolerant Rumex conglomeratus, for example, sediment adventitious roots replace 44 % of its primary root system after 2 weeks of flooding to just above the soil surface (Laan et al., 1989). When M. brownii and C. coronopifolia were flooded, aquatic adventitious roots replaced almost the entire sediment root system, contributing over 85 % of the total root dry mass, except in completely submerged M. brownii, where aquatic roots were approx. 65 % of the total root dry mass (Table 3). This extensive growth of aquatic roots into illuminated floodwaters by C. coronopifolia and M. brownii demonstrates that unlike many other species [wheat (Burström, 1960), rice (Ohno and Fujiwara, 1967), cress (MacDonald and Gordon, 1978) and maize (Pilet and Ney, 1978)], light does not have an inhibitory effect on root meristematic activity and growth in these perennial wetland species. It is unclear whether this lack of an inhibitory effect of light is species specific or related to these roots being adventitious, as several studies of adventitious rooting from cuttings have found conflicting adventitious root-growth responses under varied light regimes (Hansen, 1976; Fuernkranz et al., 1990; Fett-Neto et al., 2001; Wynne and McDonald, 2002). A lack of lateral roots on the aquatic roots of both C. coronopifolia and M. brownii (our own unpublished observations) occurred regardless of light regime (expt 3), so although light can impede lateral root initiation (Furuya and Torrey, 1964; Feldman, 1984), this was not likely to have been the cause for roots of the two species in the present study. Moreover, lateral roots did not form in C. coronopifolia and M. brownii despite no P or N being added to the submergence solution, whereas for roots of other species, low nutrient levels can promote lateral root growth (Robinson, 1994; López-Bucio et al., 2003).
In both C. coronopifolia and M. brownii, aquatic root growth into even a dimly illuminated environment (4·7 ± 2·4 µmol m−2 s−1; short maximum of 20 µmol m−2 s−1 near midday) resulted in chlorophyll synthesis and capacity for underwater PN (Fig. 3). Presumably aquatic roots also receive carbohydrate inputs from the shoot, potentially explaining the higher soluble-sugar concentration in aquatic roots of partially submerged plants relative to that of completely submerged individuals (Table 1); since shoots in air typically have high PN compared with rates when completely submerged, sugar translocation into aquatic roots of completely submerged plants is comparatively low (Rich et al., 2011). The aquatic roots of partially submerged plants had lower underwater PN rates than those of completely submerged plants (Fig. 2); however, the high PN rate of aerial tissues compared with underwater PN (Mommer et al., 2005) could result in higher overall plant carbohydrates in partially submerged plants than in completely submerged plants. The higher soluble sugars in aquatic roots of partially submerged plants may also explain the significantly longer roots developed under this treatment by both species (Table 1).
As well as photosynthesis, aquatic roots also potentially undertake some of the functions of the sediment roots, which may become inhibited under anoxic sediment conditions. Aquatic roots of both species had high porosity (Table 2) and the aerenchyma would provide a path for movement of O2 from the shoot, and also for O2 produced endogenously during photosynthesis, and/or O2 that enters from the aerobic water column (e.g. during nights). Aquatic roots could contribute to plant survival through nutrient and water uptake, and potentially in hormone production (e.g. cytokinins are produced in roots, Torrey, 1976), although this has not been studied in aquatic roots. Sediment nutrient availability is affected by anoxia and, in combination with root dysfunction, can often result in flooded plants suffering from nutritional deficiencies (Kozlowski and Pallardy, 1984). Aquatic roots, therefore, may be able to access nutrients in the floodwaters, which may be unavailable within the hypoxic roots in anoxic sediments (Končalová, 1990; Polthanee and Changdee, 2008). Concentrations of nutrients in floodwaters can be low (Setter et al., 1987); however, the total available nutrient pool is potentially large owing to the volume of, and often flowing, floodwaters. Flooding also often affects plant–water relationships through a decrease in root hydraulic conductivity, which can result in wilting and declines in stomatal conductance (Kozlowski and Pallardy, 1984). The growth of aquatic roots could potentially help maintain a favourable water balance in emergent shoots of waterlogged and partially submerged plants, and several studies have shown a positive relationship between aquatic root growth and increased stomatal conductance of aerial leaves during flooding (Gomes and Kozlowski, 1980; Topa and Cheeseman, 1992; Batzli and Dawson, 1997; Iwanaga and Yamamoto, 2008).
In summary, both M. brownii and C. coronopifolia respond to submergence with growth of an extensive aquatic root system from submerged stems, and these new roots essentially replace the existing sediment root system. Removal of aquatic roots had negative effects on plant growth during partial and complete submergence. The present results highlight the need for more research into the functioning of aquatic roots, with comparisons to sediment roots, to elucidate the beneficial role(s) of aquatic roots that makes an energy investment into their growth worthwhile to flooded plants.
LITERATURE CITED
- Batzli JM, Dawson JO. Physiological and morphological responses of red alder and sitka alder to flooding. Physiologia Plantarum. 1997;99:653–663. [Google Scholar]
- Bergman HF. The relation of aeration to the growth and activity of roots and its influence on the ecesis of plants in swamps. Annals of Botany. 1920;34:13–33. [Google Scholar]
- Bleecker AB, Schuette JL, Kende H. Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta. 1986;169:490–497. doi: 10.1007/BF00392097. [DOI] [PubMed] [Google Scholar]
- Burström H. Influence of iron and gibberellic acid on the light sensitivity of roots. Physiologia Plantarum. 1960;13:597–615. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: Gas films improve CO2 and O2 exchange. New Phytologist. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology. 2009;36:665–681. doi: 10.1071/FP09144. [DOI] [PubMed] [Google Scholar]
- Etherington JR. Comparative studies of plant growth and distribution in relation to waterlogging. X. Differential formation of adventitious roots and their experimental excision in Epilobium hirsutum and Chamerion angustifolium. Journal of Ecology. 1984;72:389–404. [Google Scholar]
- Feldman LJ. Regulation of root development. Annual Review of Plant Physiology. 1984;35:223–242. doi: 10.1146/annurev.pp.35.060184.001255. [DOI] [PubMed] [Google Scholar]
- Fett-Neto AG, Fett JP, Goulart LWV, Pasquali G, Termignoni RR, Ferreira AG. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiology. 2001;21:457–464. doi: 10.1093/treephys/21.7.457. [DOI] [PubMed] [Google Scholar]
- Fuernkranz HA, Nowak CA, Maynard CA. Light effects on in vitro adventitious root formation in axillary shoots of mature Prunus serotina. Physiologia Plantarum. 1990;80:337–341. [Google Scholar]
- Furuya M, Torrey JG. The reversible inhibition by red and far-red light of auxin-induced lateral root initiation in isolated pea roots. Plant Physiology. 1964;39:987–991. doi: 10.1104/pp.39.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes ARS, Kozlowski TT. Growth responses and adaptations of Fraxinus pennsylvanica seedlings to flooding. Plant Physiology. 1980;66:267–271. doi: 10.1104/pp.66.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. Adventitious root formation induced by gibberellic acid and regulated by the irradiance to the stock plants. Physiologia Plantarum. 1976;36:77–81. [Google Scholar]
- Hook DD, Brown CL, Korxanik PP. Lenticel and water root development of swamp tupelo under various flooding conditions. Botanical Gazette. 1970;131:217–224. [Google Scholar]
- Islam MA, Macdonald SE. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees. 2004;18:35–42. [Google Scholar]
- Iwanaga F, Yamamoto F. Effects of flooding depth on growth, morphology and photosynthesis in Alnus japonica species. New Forests. 2008;35:1–14. [Google Scholar]
- Jackson MB, Drew MC. Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT, editor. Flooding and plant growth. London: Academic Press; 1984. pp. 47–111. [Google Scholar]
- Jackson WT. The role of adventitious roots in recovery of shoots following flooding of the original root systems. American Journal of Botany. 1955;42:816–819. [Google Scholar]
- Jat RL, Dravid MS, Das DK, Goswami NN. Effect of flooding and high soil water condition on root porosity and growth of maize. Journal of the Indian Society of Soil Science. 1973;23:291–297. [Google Scholar]
- Javier RR. Effects of adventitious root removal on the growth of flooded tropical pasture legumes Macroptilium lathyroides and Vigina luteola. Annals of Tropical Research. 1985;7:12–20. [Google Scholar]
- Končalová H. Anatomical adaptations to waterlogging in roots of wetland graminoids: limitations and drawbacks. Aquatic Botany. 1990;38:127–134. [Google Scholar]
- Kozlowski TT. Extent, causes, and impacts of flooding. In: Kozlowski TT, editor. Flooding and plant growth. London: Academic Press; 1984. pp. 1–8. [Google Scholar]
- Kozlowski TT, Pallardy SG. Effect of flooding on water, carbohydrate, and mineral relations. In: Kozlowski TT, editor. Flooding and plant growth. London: Academic Press; 1984. pp. 165–193. [Google Scholar]
- Kramer PJ. Causes of injury to plants resulting from flooding of the soil. Plant Physiology. 1951;26:722–736. doi: 10.1104/pp.26.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan P, Berrevoets MJ, Lythe S, Armstrong W, Blom CWPM. Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. Journal of Ecology. 1989;77:693–703. [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- MacDonald IR, Gordon DC. The regulation of root growth in cress seedlings by light and gravity. Journal of Experimental Botany. 1978;29:1051–1058. [Google Scholar]
- Marchant NG, Wheeler JR, Rye BL, Bennett EM, Lander NS, Macfarlane TD. Flora of the Perth region. Western Australian Herbarium, Perth, Western Australia: Department of Agriculture; 1987. [Google Scholar]
- Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJW. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiology. 2005;139:497–508. doi: 10.1104/pp.105.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody ML, Les DH. Phylogenetic systematics and character evolution in the angiosperm family Haloragaceae. American Journal of Botany. 2007;94:2005–2025. doi: 10.3732/ajb.94.12.2005. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Fujiwara A. Photoinhibition of elongation growth of roots in rice seedlings. Plant and Cell Physiology. 1967;8:141–150. [Google Scholar]
- Osundina MA, Osonubi O. Adventitious roots, leaf abscission and nutrient status of flooded Gmelina and Tectona seedlings. Tree Physiology. 1989;5:473–483. doi: 10.1093/treephys/5.4.473. [DOI] [PubMed] [Google Scholar]
- Patterson KJ. A note on the aquatic vegetation of farewell spit, New Zealand. Tane. 1978;24 [Google Scholar]
- Pezeshki SR. Wetland plant responses to soil flooding. Environmental and Experimental Botany. 2001;46:299–312. [Google Scholar]
- Pilet PE, Ney D. Rapid, localized light effect on root growth in maize. Planta. 1978;144:109–110. doi: 10.1007/BF00385015. [DOI] [PubMed] [Google Scholar]
- Polthanee A, Changdee T. Influence of adventitious root removing and timing of fertilizer application in flooded soil on growth, yield and N, P, K uptake of kenaf (Hibiscus cannabinus L.) under greenhouse and field conditions. Asian Journal of Plant Sciences. 2008;7:352–359. [Google Scholar]
- Raskin I. A method for measuring leaf volume, density, thickness, and internal gas volume. Hortscience. 1983;18:698–699. [Google Scholar]
- Rich SM, Ludwig M, Colmer TD. Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata) Plant, Cell & Environment. 2008;31:1007–1016. doi: 10.1111/j.1365-3040.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Rich SM, Ludwig M, Pedersen O, Colmer TD. Aquatic adventitious roots of the wetland plant Meionectes brownii can photosynthesize: implications for root function during flooding. New Phytologist. 2011;190:311–319. doi: 10.1111/j.1469-8137.2010.03524.x. [DOI] [PubMed] [Google Scholar]
- Robinson D. The responses of plants to non-uniform supplies of nutrients. New Phytologist. 1994;127:635–674. doi: 10.1111/j.1469-8137.1994.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Setter T, Kupkanchanakul T, Pakinnaka L, Aguru Y, Greenway H. Mineral nutrients in floodwater and floating rice growing at water depths up to two metres. Plant and Soil. 1987;104:147–150. [Google Scholar]
- Shimamura S, Yoshida S, Mochizuki T. Cortical aerenchyma formation in hypocotyl and adventitious roots of Luffa cylindrica subjected to soil flooding. Annals of Botany. 2007;100:1431–1439. doi: 10.1093/aob/mcm239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique KHM, Belford RK, Tennant D. Root:shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant and Soil. 1990;121:89–98. [Google Scholar]
- Stumm W, Morgan JJ. Aquatic chemistry. New York, NY: John Wiley and Sons; 1996. [Google Scholar]
- Suge H. Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant and Cell Physiology. 1985;26:607–614. [Google Scholar]
- Teo YH, Beyrouty CA, Norman RJ, Gbur EE. Nutrient uptake relationship to root characteristics of rice. Plant and Soil. 1995;171:297–302. [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell & Environment. 1990;13:395–403. [Google Scholar]
- Topa MA, Cheeseman JM. Effects of root hypoxia and a low P supply on relative growth, carbon dioxide exchange rates and carbon partitioning in Pinus serotina seedlings. Physiologia Plantarum. 1992;86:136–144. [Google Scholar]
- Torrey JG. Root hormones and plant growth. Annual Review of Plant Physiology. 1976;27:435–459. [Google Scholar]
- Tsukahara H, Kozlowski T. Importance of adventitious roots to growth of flooded Platanus occidentalis seedlings. Plant and Soil. 1985;88:123–132. [Google Scholar]
- Van der Toorn J. On the ecology of Cotula coronopifolia L. and Ranunculus sceleratus L. I. Geographic distribution, habitat, and field observation. Acta Botanica Neerlandica. 1980;29:385–396. [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Annals of Botany. 1997;79:3–20. [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. Hormonal interplay during adventitious root formation in flooded tomato plants. The Plant Journal. 2010;63:551–562. doi: 10.1111/j.1365-313X.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Bogemann GM, Blom CWPM, Voesenek LACJ. Ethylene accumulation in waterlogged Rumex plants promotes formation of adventitious roots. Journal of Experimental Botany. 1996;47:403–410. [Google Scholar]
- Voesenek LACJ, Blom CWPM, Powels RHW. Root and shoot development of Rumex species under waterlogged conditions. Canadian Journal of Botany. 1989;67:1865–1869. [Google Scholar]
- Webb T, Armstrong W. The effects of anoxia and carbohydrates on the growth and viability of rice, pea and pumpkin roots. Journal of Experimental Botany. 1983;34:579–603. [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology. 1994;144:307–313. [Google Scholar]
- Wynne J, McDonald M. Adventitious root formation in woody plant tissue: the influence of light and indole-3-butyric acid (IBA) on adventitious root induction in Betula pendula. In vitro Cellular Developmental Biology – Plant. 2002;38:210–212. [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]