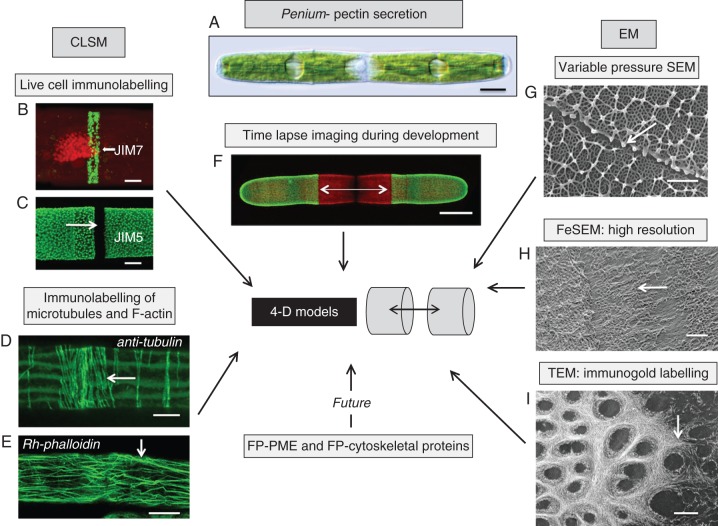
Fig. 3.
4-DI strategy of an emerging model organism, Penium margaritaceum (A). Penium is currently being used for 4-DI of pectin processing in plant cells. This alga can be live labelled with monoclonal antibodies specific for epitopes of homogalacturonans (HGs; B, C). JIM7 labels relatively high esterified HG while JIM5 labels low esterified HG. At the isthmus zone of the cell (C, arrow), the point of pre-cytokinetic wall expansion, high-esterified HG is released in a narrow band (B, arrow). As this HG is displaced outward toward both poles, it is de-esterified, most probably by the enzyme pectin methyl esterase (PME). Once de-esterified, Ca2+ binds with the HG to form a distinct lattice as noted by JIM5 labelling. The development of the wall closely corresponds with the cortical microtubule (D) and F-actin (E) networks at the isthmus. Tubulin was identified with an anti-tubulin antibody and F-actin was localized with rhodamine–phalloidin. Labelled cells are placed back in cultures and amounts of new growth can be monitored using CLSM (F, arrows). In order to elucidate further the formation of pectin, EM is also employed. To capture snapshots of rapidly developing events, cells were rapidly frozen and viewed with VPSEM (G). This allowed for structural analysis of the pectin lattice (arrow). FeSEM (H) and TEM (I) are also employed to obtain high-resolution images of the developing pectin fibrils in the isthmus (arrows). This story is only just beginning as transformed cells that are expressing FP–PME or FP–cytoskeletal proteins will yield vital dynamic information for the generation of 4-D models of pectin secretion. Scale bars: (A, F) = 17 µm, (B) = 3·5 µm, (C) = 4 µm, (D) = 3·1 µm, (E) = 14 µm, (G, H) = 250 nm, (I) = 200 nm.