Abstract
Objective
This study was to evaluate the treatment outcomes and prognostic factors of patients treated with salvage radiotherapy for the treatment of isolated lymph node recurrence of cervical cancer.
Methods
Between 1990 and 2009, 22 cervical cancer patients with lymph node recurrence who had previously undergone radical hysterectomy and pelvic lymph node dissection were treated with salvage radiotherapy with (n=18) or without (n=4) chemotherapy. Of the 22 patients, 10 had supraclavicular lymph node recurrence, 9 had para-aortic lymph node, and 3 had inguinal lymph node. The median total radiotherapy dose was 60 Gy (range, 40 to 70 Gy). Initial pathologic findings, latent period to lymph node recurrence and other clinical parameters such as squamous cell carcinoma antigen (SCC-Ag) level and concurrent chemotherapy were identified as prognostic factors for survival.
Results
The median follow-up period after salvage radiotherapy was 31.2 months (range, 12.1 to 148.9 months). The 5-year progression-free and overall survival rates of all patients were 32.7% and 30.7%, respectively. Concurrent chemoradiotherapy (p=0.009) and longer latent period to lymph node recurrence (>18 months vs. ≤18 months, p=0.019) were significant predictors of progression-free survival and SCC-Ag level at the time of recurrence (>8 ng/dL vs. ≤8 ng/dL, p=0.008) and longer latent period to lymph node recurrence (p=0.040) for overall survival. Treatment failure after salvage radiotherapy occurred in 14 (63.6%) for the 22 patients (in field, 2; out of field, 10; both in and out field, 2). Grade 3 acute skin (n=2) and hematologic toxicity (n=1) developed in 3 patients.
Conclusion
For isolated lymph node recurrence of cervical cancer, salvage radiotherapy with concurrent chemotherapy should be considered, especially in patients with a long-term progression-free period.
Keywords: Cervical cancer, Lymph nodes, Salvage therapy
INTRODUCTION
Uterine cervical cancer is one of the most common gynecologic cancers in Korea and has been associated with an excellent tumor control rate and favorable prognosis after either radiotherapy (RT) or radical hysterectomy and pelvic lymph node (LN) dissection in early stages. In Korea, these patients are usually treated via radical hysterectomy and pelvic LN dissection. However, approximately 20-40% of patients will develop recurrences [1,2], even though adjuvant whole pelvic RT with or without chemotherapy is carried out. In patients who received post operative RT for adverse pathological features, distant metastasis is the principal pattern of failure, ranging from 34% to 100% in the literature [3]. Among the patients with distant failure, LN metastasis including para-aortic lymph node (PALN) and supraclavicular lymph node (SCL) are common sites of recurrence [4-9].
For these LN recurrences, no specific treatment modality has been established. However, several recent reports have demonstrated that aggressive multimodal treatment, such as concurrent chemoradiotherapy (CCRT) and the use of advanced RT technologies e.g., (stereotactic body radiation therapy, SBRT) lead to better survival outcomes than chemotherapy alone or conventional RT techniques [10-14].
The present analysis was conducted in order to evaluate the treatment outcomes according to various variables, including squamous cell carcinoma antigen level (SCC-Ag) [15,16], initial surgical findings, and treatment factors such as salvage RT dose, concurrent chemotherapy in patients treated with salvage RT for isolated LN recurrence of cervical cancer at our hospital.
MATERIALS AND METHODS
1. Patient characteristics
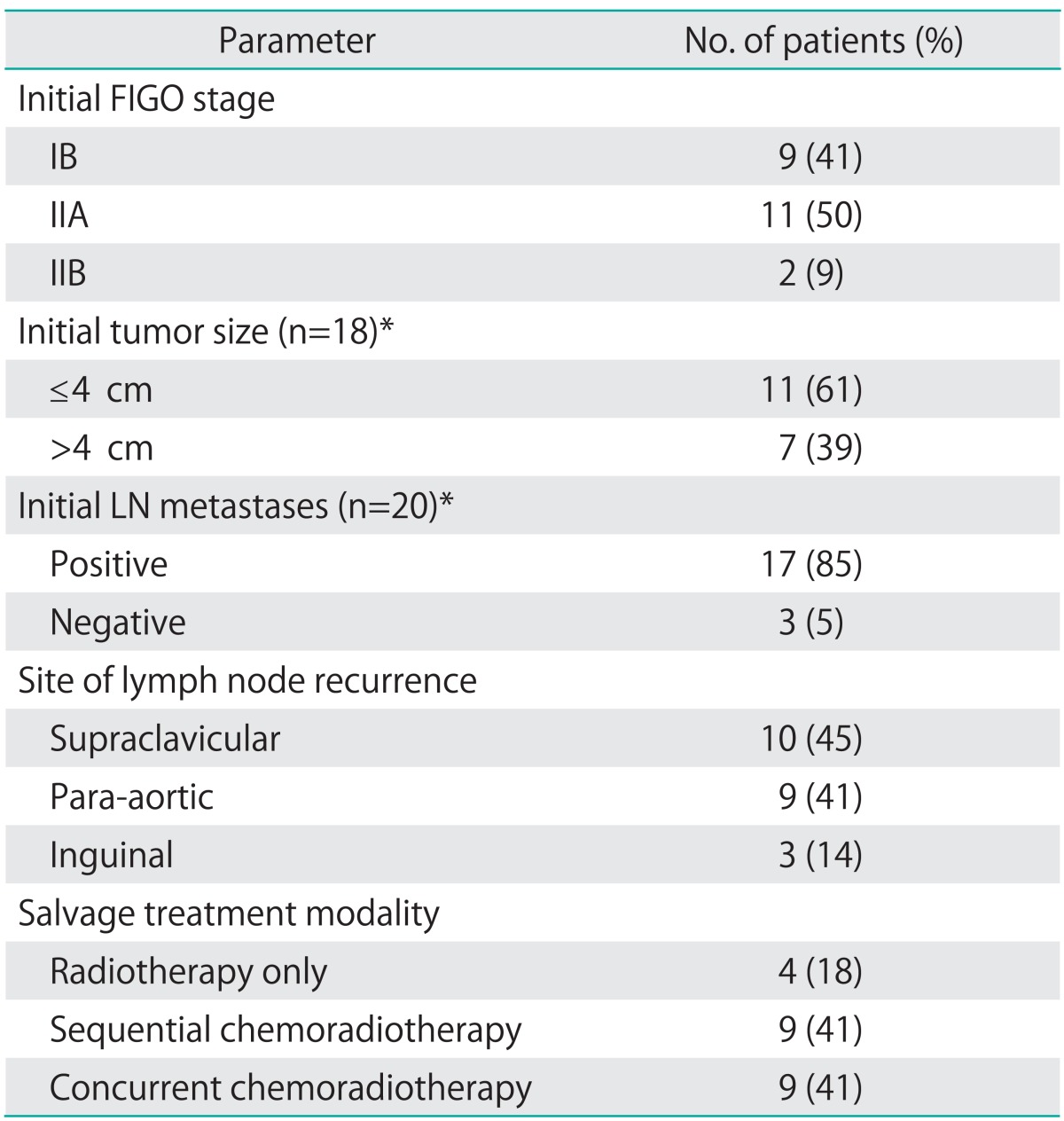
Between August 1990 and January 2011, 22 patients with isolated LN recurrence who had initially been treated with radical hysterectomy and pelvic LN dissection for cervical cancer were retrospectively reviewed. Baseline patient characteristics are summarized in Table 1. The main surgery type was radical hysterectomy (86%, 19/22) and 3 patients underwent modified radical hysterectomy. Excluding 2 patients who received radical surgery at an outside hospital, most of the patients in the study (85%, 17/20) evidenced regional LN metastasis at the initial pathologic findings. All patients received adjuvant therapy according to their initial pathologic findings. Among these patients, 10 received adjuvant chemoradiotherapy (CRT), 11 received adjuvant RT, and 1 received adjuvant chemotherapy only. Univariate and multivariate analysis were conducted to identify the correlations between survival and various variables, including initial surgical findings (tumor size and LN metastases), no evidence of disease (NED) period (≤18 months vs. >18 month), SCC-Ag level at recurrence (<8 ng/mL vs. ≥8 ng/mL), salvage RT dose (≤60 Gy vs. >60 Gy) and concurrent chemotherapy (yes vs. no). As the size of LN at the time of recurrence was not exactly evaluated due to lack of imaging study in some patients, we did not include LN size for analysis as a prognostic factor.
Table 1.
Patient characteristics (n=22)
FIGO, International Federation of Gynecology and Obstetrics.
*Available data.
2. Treatment for isolated LN recurrence
An isolated LN recurrence was defined as unequivocally enlarged LN by imaging techniques such as CT and/or positron emission tomography with [18F]-fluorodeoxyglucose (FDG-PET) scans and physical examination with no systemic metastasis at the time of recurrence. A total of 22 patients had isolated LN recurrence after radical surgery. Of these patients, SCL (n=10) and PALN (n=9) recurrence made up the major portion and 3 patients evidenced inguinal LN recurrence. The median time to recurrence from initial treatment was 29.2 months (range, 10.0 to 103.3 months).
CRT was applied to 18 patients, and 4 patients received RT alone. Of the patients who received CRT, 9 patients had sequential CRT and 9 patients received CCRT. Various chemotherapy regimens were used (5-fluorouracil [5-FU]+cisplatin, 11; taxol+carboplatin, 4; paclitaxel+carboplatin, 3). The median salvage RT dose was 60 Gy (range, 41 to 70 Gy) and 1.8 or 2 Gy dose per fractions was mainly used.
PALN recurrence was treated with a megavoltage X-ray using parallel opposite anterior/posterior fields. The radiation field encompassed the gross recurrent PALN with the superior margin at the upper end of the L1 body. The inferior margin was located between L5 and S1. In case of the previous pelvic RT, the lower border was matched to the superior edge of the previous pelvic port with 2-cm gap. A margin of 2 cm was given to gross disease. Radiation up to 41.4-50.4 Gy was delivered, and then boost RT by cone-down field was given to grossly enlarged nodes, up to a total dose of 60 Gy. For SCL recurrence, external beam radiation therapy (EBRT) was delivered using a megavoltage X-ray through the parallel-opposed ports. The lower border was lower border of the clavicle, and the upper border was extend on or about to the level of C4 to cover SCL area. The medial field edge extended to the area of the lateral vertebral body and laterally beyond the humeral head. Boost RT was given to palpable nodes with 2 cm margin, up to a total dose of 70 Gy. For inguinal LN recurrence, a minimum 2-cm margin around gross tumor was used for clinical target volume with radiation dose up to 66.6 Gy.
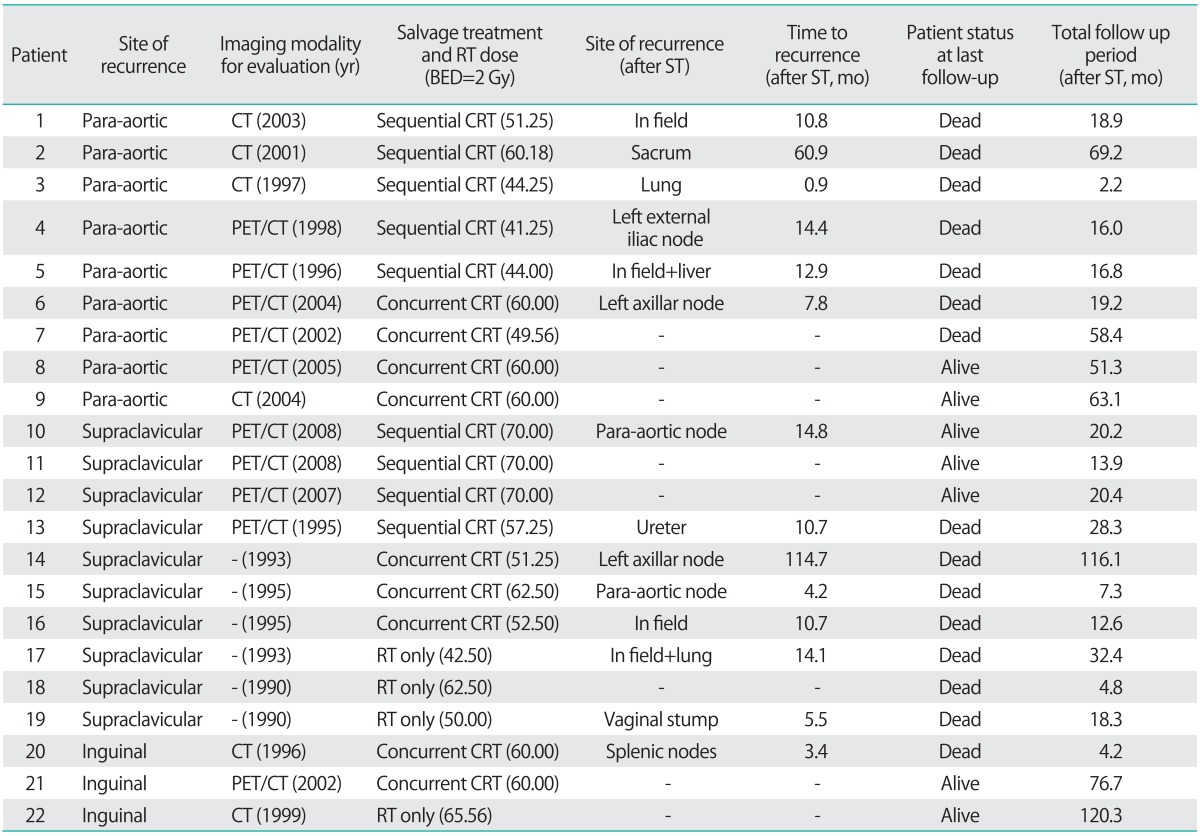
Because various radiation doses per fraction scheme (1.8 to 3.0 Gy) were used, we calculated equivalent total doses when applied in 2 Gy per fractions (Table 2).
Table 2.
Result of salvage treatment according to specific recurrence site
BED, biological equivalent dose; CRT, chemoradiotherapy; RT, radiotherapy; ST, salvage therapy.
3. Toxicity evaluation
Acute toxicity was defined as toxicity occurring within 90 days after salvage therapy and any toxicity occurring more than 90 days after treatment was regarded as late toxicity. Due to different isolated LN recurrence sites, toxicities were evaluated according to each treatment site. For SCL treatment, skin toxicity was mainly noted and evaluated. Likewise, gastrointestinal toxicity was evaluated via PALN treatment. Hematologic toxicity was also evaluated in patients who received chemotherapy either sequentially or concurrently. Treatment-related acute and late toxicity was assessed according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
4. Statistic analysis
Progression-free survival (PFS) and overall survival (OS) curves were calculated in accordance with the Kaplan-Meier method. Using the log-rank test, the prognostic factors influencing survival were analyzed. p-values of less than 0.05 were considered statistically significant. The Cox proportional hazard regression model with the stepwise forward procedure was used in multivariate analysis. All analysis was carried out using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The study protocol was reviewed and approved by the institutional review board of our Hospital. The recommendations of the Declaration of Helsinki for biomedical research involving human subjects were also reflected.
RESULTS
1. Treatment results and prognostic factors
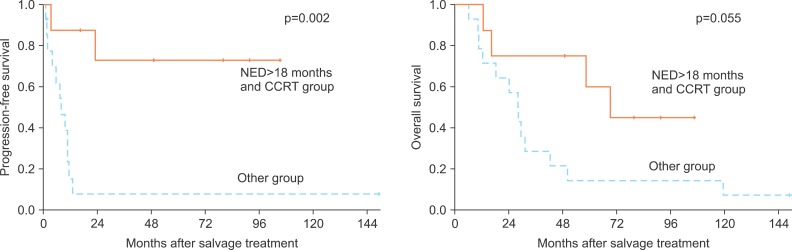
The median patient age was 51 years (range, 32 to 65 years) and the median follow-up duration from initial surgery was 31.2 months (range, 12.1 to 148.9 months). The 5-year PFS and OS rates for all 22 patients were 32.7% and 30.7%. Univariate analysis demonstrated that a NED period of more than 18 months (5-year PFS, 16.7 vs. 34.1%; p=0.005), less than 8 ng/mL SCC level at the time of recurrence (23.4% vs. 66.7%; p=0.001), more than a 60 Gy RT dose (10% vs. 53%; p=0.039) and concurrent chemotherapy (8.3% vs. 64.8%; p=0.01) were statistically significant with regard to PFS. Of these statistically significant factors, NED period (p=0.019) and CCRT (p=0.009) were significant on the Cox regression test. For overall survival, NED period of more than 18 months (5-year OS, 0% vs. 40.3%; p=0.017) and less than 8 ng/mL SCC level at the time of recurrence (0% vs. 53.3%; p=0.001) were statistically significant on univariate analysis. In multivariate analysis for OS, those two factors also evidenced clinical significance. Initial surgical findings such as tumor size and LN metastasis were not associated with PFS or OS. Of these analyzed variables, patients who had both a NED period of more than 18 months and those who received CCRT (36%, 8/22) evidenced a 72.9% 5-year PFS rate and a 60% 5-year OS rate (Fig. 1). According to recurrent sites, LN recurrence at the para-aortic or inguinal region was associated with superior 5-year PFS and OS rates relative to the SCL region. The results of salvage treatment according to specific sites are summarized in Table 2.
Fig. 1.
Progression-free and overall survival of no evidence of disease (NED) >18 months and concurrent chemoradiotherapy (CCRT) group.
2. Toxicity
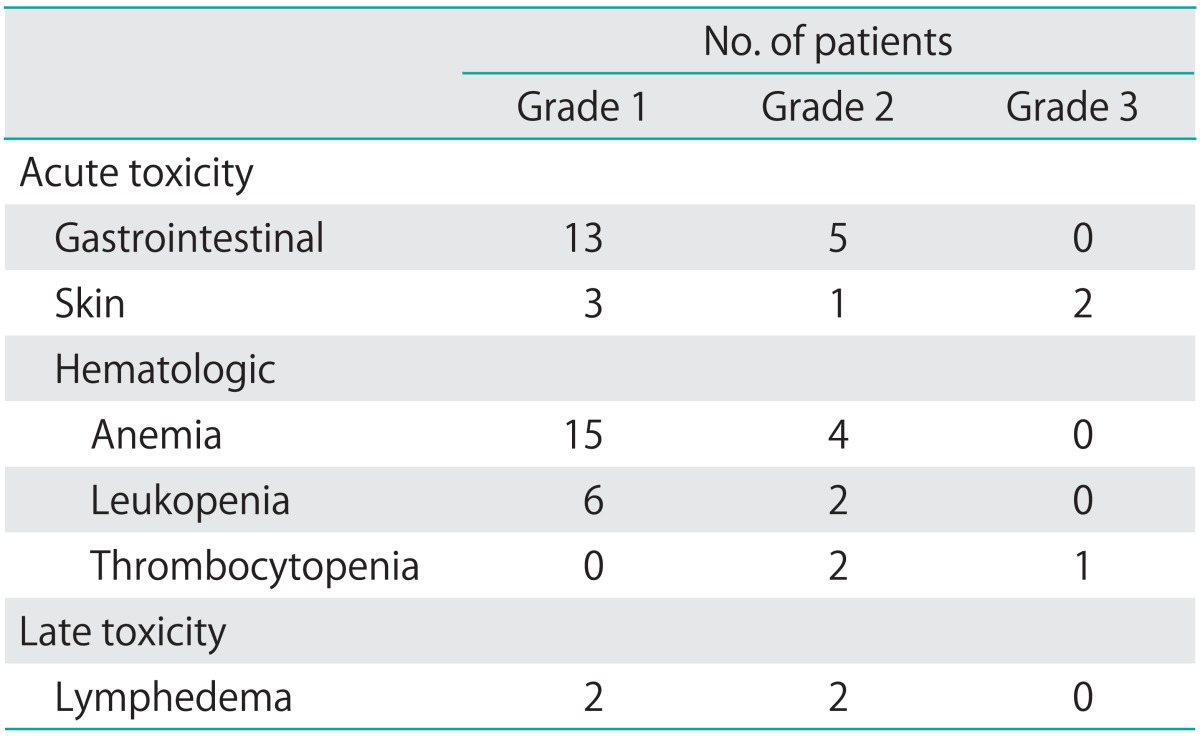
Using CTCAE v3.0, acute and late toxicity were evaluated during and after salvage treatment (Table 3). Grade 3 acute skin toxicity developed in 2 patients with SCL treatment. One patient experienced grade 3 thrombocytopenia during CCRT. Gastrointestinal toxicity mainly developed in patients with PALN treatment and acute grade 1-2 hematologic toxicity occurred in patients with chemotherapy. For late toxicity, grade 2 lymphedema developed in 2 patients.
Table 3.
Acute and late toxicity according to Common Toxicity Criteria for Adverse Events v.3.0 grading scale
3. Patterns of failure after salvage RT
A total of 14 patients evidenced disease progression after salvage treatment. We evaluated the patterns of failure according to salvage RT field and specific isolated LN site. Most of the failures (12/14) occurred out of the RT fields, and 4 patients had in-field recurrence after salvage RT. Of the patients who experienced out-of-RT-field recurrence, only 4 patients had FDG-PET scans at the time of recurrence. Also, 4 patients experienced out-of-field recurrence within 6 months after salvage treatment, and all these patients had not undergone FDG-PET before treatment (Table 2). Most of the patients who had disease progression after salvage treatment died at the final follow-up (92.8%, 13/14).
DISCUSSION
LN recurrence in cervical cancer is frequently associated with simultaneous distant metastasis and usually with poor outcomes. However, isolated LN recurrence is associated with relatively good prognosis. Several studies reported the outcomes of salvage RT with chemotherapy in patients with isolated LN recurrence after primary radical RT. Chou et al. [13] reported isolated PALN recurrence after primary irradiation could be treated with cisplatin-based CCRT and showed a 30.8% 5-year survival rate. Additionally, Kim et al. [17] demonstrated that hyperfractionated RT with concurrent chemotherapy to PALN recurrence in cervical cancer could be employed as an effective treatment modality with no significant toxicity and 19% of 3-year survival rate. Singh et al. [12] demonstrated that salvage concurrent full-dose CRT applied to isolated PALN recurrence affords excellent survival in patients who did not have the classic symptom triad and more than 24 months' recurrence time from initial treatment.
Unlike the above studies, which focused on isolated PALN recurrence after primary radical RT, there have been a few studies which reported the treatment outcomes of salvage RT for isolated LN recurrence after primary radical surgery. This study included any recurrent sites of isolated LN, and these included PALN (n=9), SCL (n=10), and inguinal LN (n=3) metastases. Even though this study consisted of SCL recurrences for analysis, survival outcomes were similar to or slightly better than those of previously reported studies, achieving more than a 20% 5-year PFS (SCL, 22.0%; PALN, 33.3%) rate. Including isolated inguinal LN recurrence, total 5-year PFS and OS survival rate was 32.7% and 30.7%.
A wide time-range of the onset of isolated LN recurrence was noted in our study (10.0 to 103.3 months). Most of the patients (17/22) experienced recurrence >18 months after primary radical surgery, and these patients had better prognoses. Also, the addition of concurrent chemotherapy rather than RT alone or sequential chemotherapy was associated with superior survival, which might be a result of the role of chemotherapy as a radiation enhancer, as in the setting of primary radical treatment. Patients who both had an NED period of more than 18 months and had also received CCRT (8/22) may achieve a long-term PFS without cancer progression (5-year PFS, 72.9%). Based on our results, salvage RT with concurrent chemotherapy should be considered, especially in patients with a long-term progression-free period.
SCC-Ag is known to be a useful marker for the detection of recurrence after radical treatment, and the SCC-Ag level at the time of recurrence has important prognostic significance [6,17]. Although the cut-off SCC-Ag level in this study differed from that of other studies, this study also showed that SCC-Ag level at recurrence was an important prognostic factor for overall survival. Therefore, it would be desirable to regard SCC-Ag as a follow-up tool for the detection of recurrence and the execution of early salvage treatment.
This study analyzed whether initial pathologic findings such as tumor size and LN metastases affect salvage treatment results. However, no significant relationship was detected between initial pathologic findings and survival outcomes in this study. This finding indicates that surgical pathologic findings might merely be a prognostic factor at the time of initial treatment.
As for patterns of failure after salvage RT, this study demonstrated that only small numbers of patients had in-field recurrence and the principal pattern of failure observed is systemic relapse of disease. Previously conducted studies [18,19] demonstrated that FDG-PET is a valuable imaging modality for determining the extent of tumor spread or disease recurrence. In our study group, systemic work-up with FDG-PET scan was carried out in 10 patients (45%, 10/22). Five out of 10 patients who had undergone FDG-PET at the time of recurrence showed treatment failure after salvage RT, and the result is compared with those of the patients who had not (CT only, 4/6; no image, 5/6). Also, four patients who recurred within 6 months after salvage treatment had not undergone FDG-PET scan at the time of recurrence. These findings indicate these patients may have undiagnosed recurrence and synchronous metastasis at the time of salvage treatment. The extent of disease recurrence is not only an important prognostic factor in recurrent cervical cancer, but may also be employed as a determining factor for what salvage treatment modality can be applied. For single isolated LN recurrences in cervical cancer, modern RT and concurrent chemotherapy may be used, and superior treatment outcomes can be achieved. However, patients who had multiple metastases at the time of recurrence may require palliative chemotherapy as opposed to definitive CRT. Our institution adopted the FDG-PET scan in 1994. Due to long-term follow-up of whole patient groups, more than half of the patients received salvage treatment without pre-treatment whole body PET scan, and thus synchronous distant metastasis could not be completely excluded.
Besides the implementation of FDG-PET, our study is limited in several regards. First, this retrospective study involved a relatively small number of patients and the sites of isolated LN recurrence were different. The patient characteristics evidenced different survival rates according to the site of LN recurrence. Secondly, nearly half of the study group (63%, 10/22) received suboptimal radiation dose of less than 60 Gy, especially in the 1990s and early 2000s. Actually, the range of radiation dose given to all 4 patients who experienced in-field failure was 45 to 50.4 Gy. Nowadays, we deliver higher dose to the gross LN lesion and reduce the normal organ toxicity. Furthermore, in the era of SBRT, we are able to provide a much higher dose to the tumor with a steep dose gradient, reduce the normal tissue damage and achieve a relatively short-term treatment period compared to conventional radiation techniques [14]. Hence, these advances in RT technique may constitute a more efficacious way to treat single isolated LN metastases in gynecological cancer.
In conclusion, salvage RT with concurrent chemotherapy applied in cases of isolated LN recurrence in cervical cancer can achieve long-term PFS and OS. NED periods of more than 18 months, SCC levels below 8 ng/mL at the time of LN recurrence, and concurrent chemotherapy were identified as important factors for good prognosis. Considering that treatment-related toxicity was well tolerated, more intensive follow-up with advanced imaging modality and salvage treatment with highly conformal RT technique for the treatment of isolated LN recurrences in cervical cancer will be required for better survival.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Brady LW, Perez CA, Bedwinek JM. Failure patterns in gynecologic cancer. Int J Radiat Oncol Biol Phys. 1986;12:549–557. doi: 10.1016/0360-3016(86)90062-3. [DOI] [PubMed] [Google Scholar]
- 2.Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24:197–204. doi: 10.1016/0360-3016(92)90671-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Kim HJ, Hong S, Wu HG, Ha SW. Post-hysterectomy radiotherapy in FIGO stage IB-IIB uterine cervical carcinoma. Gynecol Oncol. 2005;96:407–414. doi: 10.1016/j.ygyno.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Rotman M, Pajak TF, Choi K, Clery M, Marcial V, Grigsby PW, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas: ten-year treatment results of RTOG 79-20. JAMA. 1995;274:387–393. [PubMed] [Google Scholar]
- 5.Sakurai H, Mitsuhashi N, Takahashi M, Akimoto T, Muramatsu H, Ishikawa H, et al. Analysis of recurrence of squamous cell carcinoma of the uterine cervix after definitive radiation therapy alone: patterns of recurrence, latent periods, and prognosis. Int J Radiat Oncol Biol Phys. 2001;50:1136–1144. doi: 10.1016/s0360-3016(01)01573-5. [DOI] [PubMed] [Google Scholar]
- 6.Niibe Y, Kazumoto T, Toita T, Yamazaki H, Higuchi K, Ii N, et al. Frequency and characteristics of isolated para-aortic lymph node recurrence in patients with uterine cervical carcinoma in Japan: a multi-institutional study. Gynecol Oncol. 2006;103:435–438. doi: 10.1016/j.ygyno.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Carlson V, Delclos L, Fletcher GH. Distant metastases in squamous-cell carcinoma of the uterine cervix. Radiology. 1967;88:961–966. doi: 10.1148/88.5.961. [DOI] [PubMed] [Google Scholar]
- 8.Stock RG, Chen AS, Flickinger JC, Kalnicki S, Seski J. Node-positive cervical cancer: impact of pelvic irradiation and patterns of failure. Int J Radiat Oncol Biol Phys. 1995;31:31–36. doi: 10.1016/0360-3016(94)00391-W. [DOI] [PubMed] [Google Scholar]
- 9.Huang EY, Wang CJ, Chen HC, Fang FM, Huang YJ, Wang CY, et al. Multivariate analysis of para-aortic lymph node recurrence after definitive radiotherapy for stage IB-IVA squamous cell carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 2008;72:834–842. doi: 10.1016/j.ijrobp.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Higginson DS, Morris DE, Jones EL, Clarke-Pearson D, Varia MA. Stereotactic body radiotherapy (SBRT): technological innovation and application in gynecologic oncology. Gynecol Oncol. 2011;120:404–412. doi: 10.1016/j.ygyno.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 12.Singh AK, Grigsby PW, Rader JS, Mutch DG, Powell MA. Cervix carcinoma, concurrent chemoradiotherapy, and salvage of isolated paraaortic lymph node recurrence. Int J Radiat Oncol Biol Phys. 2005;61:450–455. doi: 10.1016/j.ijrobp.2004.06.207. [DOI] [PubMed] [Google Scholar]
- 13.Chou HH, Wang CC, Lai CH, Hong JH, Ng KK, Chang TC, et al. Isolated paraaortic lymph node recurrence after definitive irradiation for cervical carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:442–448. doi: 10.1016/s0360-3016(01)01628-5. [DOI] [PubMed] [Google Scholar]
- 14.Choi CW, Cho CK, Yoo SY, Kim MS, Yang KM, Yoo HJ, et al. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147–153. doi: 10.1016/j.ijrobp.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Bolli JA, Doering DL, Bosscher JR, Day TG, Jr, Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–173. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- 16.Rose PG, Baker S, Fournier L, Nelson BE, Hunter RE. Serum squamous cell carcinoma antigen levels in invasive cervical cancer: prediction of response and recurrence. Am J Obstet Gynecol. 1993;168:942–946. doi: 10.1016/s0002-9378(12)90850-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Kim JS, Kim SY, Kim KH, Cho MJ. Hyperfractionated radiotherapy with concurrent chemotherapy for para-aortic lymph node recurrence in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2003;55:1247–1253. doi: 10.1016/s0360-3016(02)04401-2. [DOI] [PubMed] [Google Scholar]
- 18.Ho KC, Wang CC, Qiu JT, Lai CH, Hong JH, Huang YT, et al. Identification of prognostic factors in patients with cervical cancer and supraclavicular lymph node recurrence. Gynecol Oncol. 2011;123:253–256. doi: 10.1016/j.ygyno.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–3749. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]