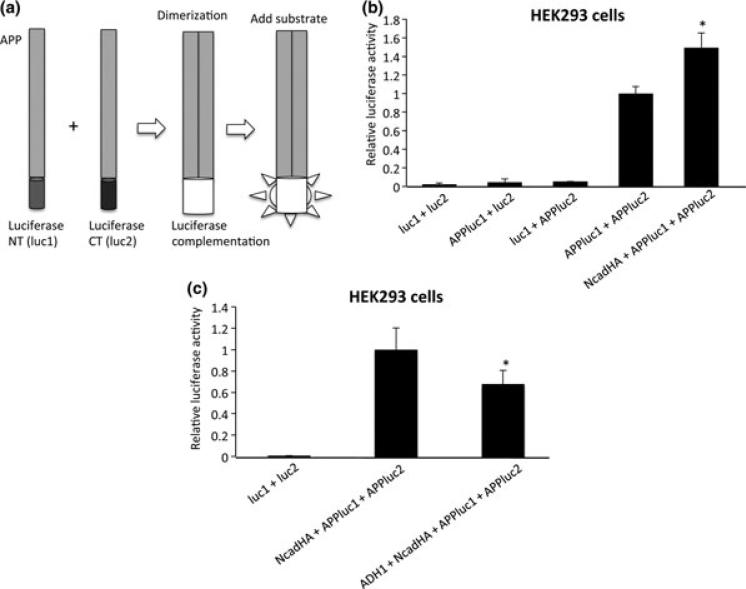
Fig. 2.
Split luciferase assay for quantitative analysis of APP cisdimerization. (a) APP was fused with either N-terminal (luc1) or C-terminal (luc2) half of humanized Gaussia luciferase. As they form dimers, they fold into active luciferase, whose activity can be visualized by the addition of luciferase substrate. (b) HEK293 cells were cotransfected with APPluc1 and APPluc2 in the presence or absence of NcadHA. Luciferase activity in NcadHA + APPluc1 + APPluc2-transfected cells showed an approximately 1.5-fold higher activity than that in APPluc1 + APPluc2-transfected cells (*p < 0.01). Conversely, luc1 + luc2, APPluc1 + luc2 and luc1 + APPluc2 did not show significant increase in luciferase activity. (luc1 + luc2 n = 6, APPluc1 + luc2 n = 6, luc1 + APPluc2 n = 3, APPluc1 + APPluc2 n = 9, NcadHA + APPluc1 + APPluc2 n = 6). (c) ADH-1 treatment inhibit APP dimerization. HEK293 cells were co-transfected with APPluc1, APP-luc2 and NcadHA. After transfection, cells were treated with 1 mg/mL of ADH-1 for 24 h. Luciferase activity was significantly decreased in cells treated with ADH-1 (*p < 0.01). (luc1 + luc2 n = 6, Ncad + APPluc1 + APPluc2 n = 6, ADH-1 + Ncad + APPluc1 + APPluc2 n = 6).