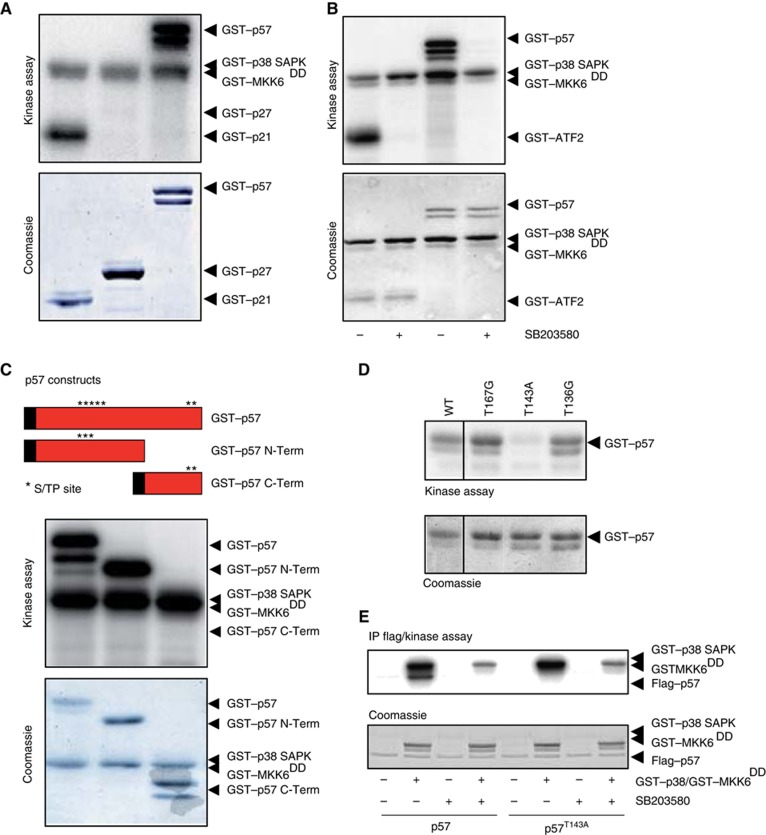
Figure 1.
p38 SAPK phosphorylates the CDKi p57 at T143 in vitro. (A) All proteins were purified from E. coli. GST–p57 was expressed as a doublet protein being the shorter a cleavage fragment of full-length GST–p57. GST–p38 SAPK phosphorylates human GST–p21 and mouse GST–p57 but not human GST–p27. (B) GST–ATF2 and GST–p57 phosphorylation is prevented by the p38 SAPK inhibitor SB203580. (C) The mouse p57 protein harbours five putative phosphorylation sites following the minimum SAPK S/TP motif clustered in two regions. A GST–p57 N-term, containing three putative sites, and a GST–p57 C-term, containing two putative sites, mutants were made and assayed in vitro in the presence of GST–p38 SAPK. The GST–p57 N-term variant is phosphorylated as well as wild-type GST–p57 whereas the GST–p57 C-term variant is not phosphorylated by the p38 SAPK. (D) The three putative p38 SAPK sites T167, T143 and T139 were mutated to either glycine or alanine and assayed in vitro. The GST–p57 T143A mutant is not phosphorylated by GST–p38 SAPK in vitro. (E) HeLa cells were transfected with Flag-tagged wild-type p57 and p57T143A. Forty-eight hours post transfections, cells were lysed and immunoprecipitated with anti-Flag agarose beads. Immunoprecipitates were assayed in vitro with GST–p38 SAPK in the presence or the absence of the p38 inhibitor SB203580. Only wild-type Flag–p57 was phosphorylated by p38 SAPK. Representative kinase assays and coomassie blue stained gels are shown.