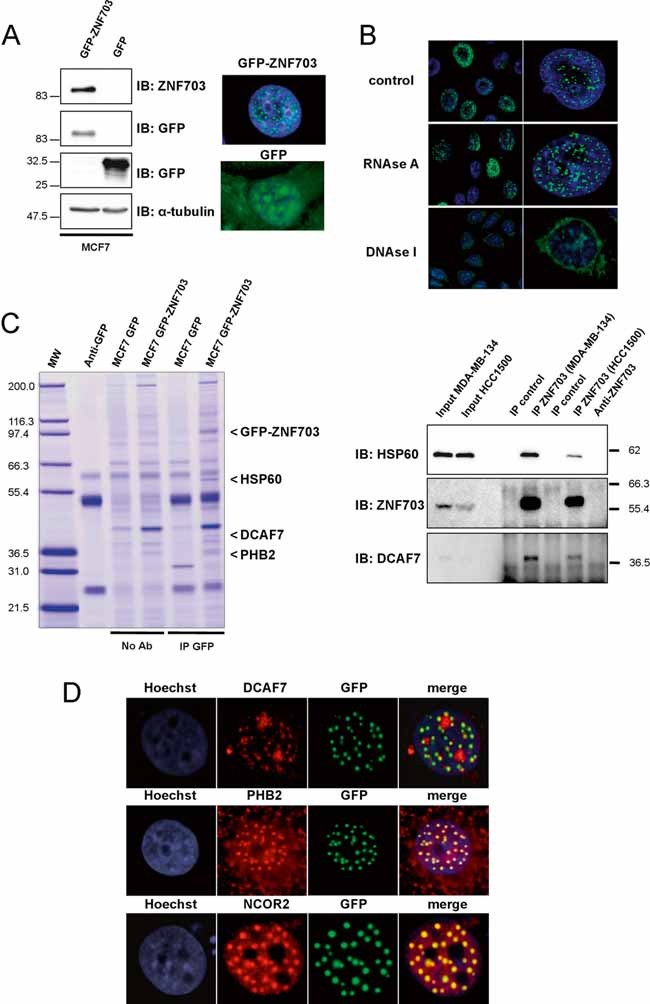
Immunoblotting and immunofluorescence analyses of MCF7 GFP-ZNF703 and MCF7 GFP breast cancer cell lines. Immunoblotting with anti-ZNF703 and anti-GFP antibodies identifies GFP-ZNF703 fusion protein with a molecular weight of 83 kDa. α-tubulin protein expression is shown as a control. Immunofluorescence reveals nuclear dot-like structures in MCF7 GFP-ZNF703 cells, whereas GFP is detected in all subcellular structures in MCF7 GFP cells.
GFP-ZNF703 interacts with DNA in the nucleus. MCF7 GFP-ZNF703 cells non-treated (control) or treated with RNAse A or DNAse I were processed for immunofluorescence with anti-GFP antibody. The GFP-ZNF703 signal in the nucleus is perturbed only when cells are treated with DNAse.
MCF7 cells expressing GFP and GFP-ZNF703 proteins were subjected to immunoprecipitation with anti-GFP antibody (IP GFP) or beads only (No Ab), separated by SDS-PAGE, and stained with colloidal Coomassie blue. Three co-precipitated proteins were identified by mass spectrometry: HSP60 (accession number, NP 955472); DCAF7 (NP 005819) and PHB2 (NP 001138303), respectively. The lane on the left contains molecular weight standards (MW). The interaction between ZNF703 and HSP60 or DCAF7 was confirmed by ZNF703-immunoprecipitation, followed by Western blot analysis using anti-HSP60 or anti-DCAF7 antibody. Input represents whole-cell extract from HCC1500 and MDA-MB-134.
MCF7 cells overexpressing GFP-ZNF703 were fixed and immunostained with anti-DCAF7, anti-PHB2 or anti-SMRT/NCOR2 antibody. GFP-ZNF703 (green) co-localizes in the nucleus with DCAF7, PHB2 and SMRT/NCOR2 (red). Nuclei are counterstained with Hoechst (blue).