Figure 1.
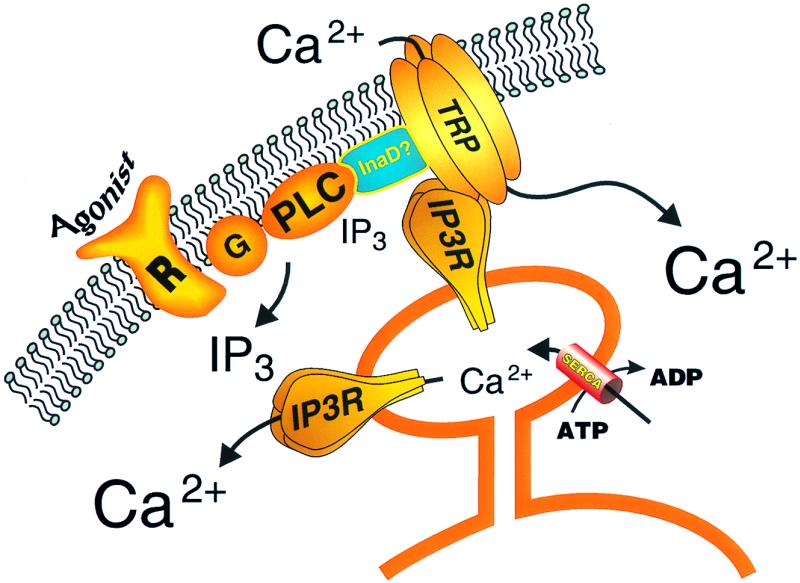
Model for regulation of capacitative calcium entry channels by IP3 receptors. Agonist activation of a surface membrane receptor (R), perhaps through a heterotrimeric G-protein (G), activates phospholipase C (PLC), leading to the production of the calcium-mobilizing messenger, IP3. IP3 releases calcium from a critical endoplasmic reticulum store. The fall in luminal calcium in this store causes a conformational change in an IP3 receptor that interacts with a TRP subunit of the capacitative calcium entry channel, causing it to open. A signaling complex containing the PLC, TRP, and IP3 receptor may require an as yet uncharacterized scaffolding protein, perhaps similar to InaD. The close spatial arrangement of this signaling complex results in a constant supply of IP3 from basal PLC activity for the IP3 receptor, such that IP3 levels are not normally limiting; rather, it is depletion of calcium from the specific intracellular store that provides the critical signal for channel activation.
