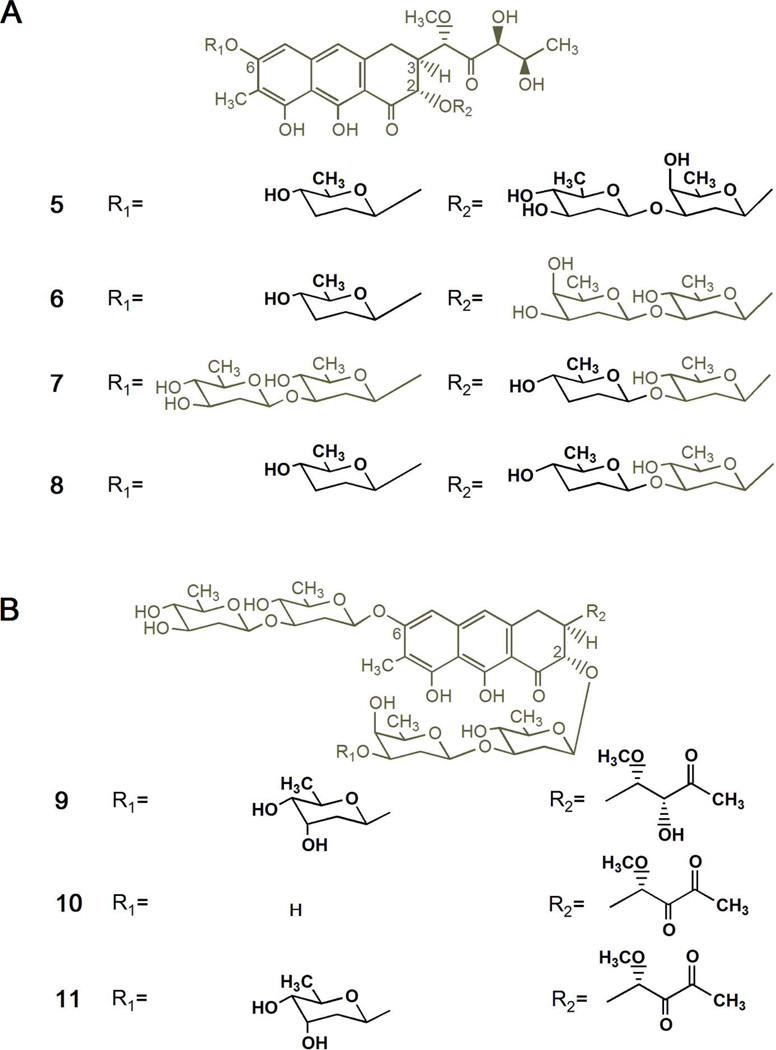
Figure 2.
Chemical structures of new mithramycin analogues generated by combinatorial biosynthesis. (A) Compounds with modified glycosylation patterns produced by S. argillaceus M7C1-pFL845: dideolivosyl-6-β-d-amicetosyl-demycarosyl-2-O-β-d-oliosyl-3C-β-d-olivosyl-mithramycin (5), dideolivosyl-6-β-d-amicetosyl-demycarosyl-mithramycin (6), deoliosyl-demycarosyl-3C-β-d-amicetosyl-mithramycin (7), and dideolivosyl-6-β-d-amicetosyl-deoliosyl-demycarosyl-3C-β-d-amicetosyl-mithramycin (8). (B) compounds with modified glycosylation pattern and different structure of the 3-carbon side chain produced by S. argillaceus M3W1-pMP*3BII: demycarosyl-3D-β-d-digitoxosyl-mithramycin SK (9), demicarosyl-mithramycin SDK (10), and demycarosyl-3D-β-d-digitoxosyl-mithramycin SDK (11). Differences from 1 are highlighted in bold.