Harnessing the CD14-independent pathway for chemokine induction in severe infection leads to early neutrophil recruitment to the site of infection, enhanced bacterial clearance, and survival.
Keywords: sepsis, bacteria, LPS
Abstract
Previous studies have shown that CD14−/− mice are resistant to peritoneal infection with some clinical isolates of Escherichia coli and that this resistance is accompanied by an enhanced ability to clear the bacteria; in contrast, normal mice expressing CD14 fail to clear the bacteria, causing severe sepsis and death. The enhanced clearance in CD14−/− mice is dependent on early neutrophil recruitment to the local foci of infection in the PC. The studies described show that neutrophil recruitment in CD14−/− mice occurs as a result of the local induction of the CXCL1 and CXCL2 chemokines, KC and MIP-2. Although local induction of these chemokines also occurs in normal mice, their effects on neutrophil recruitment to the PC appear to be counterbalanced by very high levels of these chemokines in the blood of normal, but not CD14−/−, mice. Neutrophil recruitment to the PC is also inhibited in normal mice in response to LPS, which also induces high chemokine levels in the blood of normal, but not CD14−/−, mice. However, MPLA, a monophosphorylated derivative of LPS, is able to induce early neutrophil recruitment in normal mice; this is because MPLA, unlike LPS or E. coli, induces MIP-2 and KC in the PC but not in the blood of normal mice. The pretreatment of normal mice with MPLA is able to protect them from a lethal E. coli infection. Thus, stimulation of a local CD14-independent chemokine induction pathway without triggering a systemic CD14-dependent chemokine pathway can protect against severe E. coli infections.
Introduction
Sepsis, characterized by a systemic bacterial infection, is a life-threatening condition that can lead to multiorgan failure and death from septic shock [1]. The host's response to Gram-negative bacteria can result in a strong innate immune response, including induction of proinflammatory cytokines, such as TNF, IL-1, and IL-6 via the CD14/TLR4 pathway [2, 3] and a cascade of events that can lead to death during the early phases of sepsis [4–7]. Mortality is also observed in later stages of infection and has been attributed to the second phase of immune suppression that follows the initial hyperimmune response [8]. Several reports suggest that the ability to limit bacterial dissemination may be critical in preventing death from sepsis [8–10]. Importantly, previous studies from this laboratory indicate that CD14, a coreceptor for LPS, plays a key role in regulating this bacterial dissemination; CD14−/− mice show enhanced clearance of laboratory and clinical isolates of E. coli, both at the site of injection (PC) as well as in blood [11–13]. Prior studies with a laboratory strain of E. coli had shown that protection from infection and enhanced bacterial clearance were associated with an early recruitment of neutrophils to the site of infection (PC) in CD14−/− but not normal mice [12]. The studies described here were designed to elucidate the mechanism for this early neutrophil recruitment in CD14−/− mice and to determine whether a similar mechanism was operational in protecting CD14−/− mice from infection with clinical isolates of E. coli. In addition, the ability and mechanism of action of MPLA, a monophosphorylated derivative of LPS, to induce early neutrophil recruitment and protect normal mice from severe E. coli sepsis were studied.
MATERIALS AND METHODS
Mice
All mice used were 8–12 weeks of age. WT (C57BL/6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), and WT CF-1 mice were purchased from Charles Rivers Laboratories (Wilmington, MA, USA). The CD14−/− mice (129J-Cd14tm1) were backcrossed onto the C57BL/6 background for 11 generations [11]. Mice were provided with nonsterile laboratory chow (Harlan Teklad, Madison, WI, USA) and water ad libitum. All animal studies were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and were preapproved by the Animal Use Committee for The City College of New York.
Bacterial strains
E. coli isolate 69 is an isolate from a sepsis patient [13, 14]. E. coli RS218D is an isogenic K1 deletion mutant generated from E. coli strain E44, a spontaneous rifampin-resistant mutant isolated from the cerebrospinal fluid of a neonate with meningitis [13, 15].
Culture of bacteria
E. coli isolate 69 was grown in TSB or TSA (Difco, Detroit, MI, USA). E. coli RS218D was grown in TSB or TSA, supplemented with 50 μg/ml streptomycin and 40 μg/ml chloramphenicol. Individual isolates were grown in 5 ml TSB with (RS218D) or without (isolate 69) antibiotics after inoculation of a single colony and incubated at 37°C overnight in an orbital shaker. An aliquot of the overnight culture (0.5 ml) was used to inoculate 24.5 ml fresh TSB, with or without antibiotics, and incubated for 2 h at 37°C with shaking. The culture was concentrated by centrifugation, washed, and resuspended in 12 ml sterile saline. The number and viability of the bacteria were determined using a LIVE/DEAD BacLight kit (Molecular Probes, Eugene, OR, USA). The culture was diluted to the required dose in DPBS (Invitrogen, Carlsbad, CA, USA), and 0.2 ml was injected (i.p.) into the mouse. The dose was confirmed by plating dilutions of the culture used for injection on TSA, with or without antibiotics.
Survival studies
A dose of 1.9 × 106 cfu/gbw (isolate 69) or 2.5–4.7 × 106 cfu/gbw (RS218D) was injected (i.p.), and the mice were monitored for a period of 6–7 days.
Bacterial clearance
At various time-points after injection (i.p) of E. coli RS218D (doses described above), mice were killed with CO2; blood was collected by exsanguination in heparin-rinsed syringes; and the peritoneal fluid was collected by lavage with 3.0 ml RPMI 1640, containing 10 mM Hepes buffer and supplemented with 1% FBS. Serial dilutions of blood and PLF were plated on TSA with antibiotics. The number of residual live bacteria in the PC was determined by normalizing the viable counts with the volume of medium injected.
Cell recruitment and chemokine measurements
The PLF was centrifuged (600 g) for 10 min at 4°C. The supernatant was aliquoted and stored at −80°C. The levels of MIP-2 and KC in the blood and PLF samples were determined by ELISA with Duoset kits (R&D Systems, Minneapolis, MN, USA). The total amounts of chemokines in the PC were determined by normalizing for the volume of medium injected. The cell pellet was resuspended in 1.0 ml medium (described above for PLF). Total counts and differentials of cytospin smears stained with Camco differential stain (Cambridge Diagnostic Products, Fort Lauderdale, FL, USA) were determined by light microscopy. The percentage of PMNs was determined after counting 400 cells/slide. Total neutrophils recruited to the PC were calculated and normalized for the volume of medium injected for lavage of PC. Data were plotted as percent PMN or as total PMNs recruited to the PC.
Chemokine induction by resident peritoneal cells induced in vitro
The resident peritoneal cells were collected by lavage, the PLF was centrifuged as above, and the cell pellet was washed (with RPMI 160, with 20 mM Hepes buffer and 1% autologous serum). Washed cells were resuspended in the same medium (0.5 ml) at a density of 1 × 106 cells/well and exposed to increasing concentrations of bacteria (2.8×103 cfu–2.8×108 cfu) for 2.5 h at 37°C with 5% CO2. Cell supernatants were analyzed for the chemokines MIP2 and KC by ELISA.
Pretreatment with antibodies
All antibodies were resuspended in DPBS before injection. Neutrophil-depleting antibodies (anti-Ly6G, clone 1A8) or its isotype control (BD Biosciences, San Jose, CA, USA) were injected (i.p., 50 μg/200 μl) to deplete PMNs 18 h prior to injection of bacteria [16]. Anti-CXCR2 (mAb 2164) or its isotype control (R&D Systems; 80 μg/200 μl) was injected (i.p.) 45 min after the injection of bacteria [17]. For MPLA studies, E. coli was injected (i.p.) into normal mice, 2.5 h following i.p. injection of MPLA (36 ng/gbw), alone or in a mixture of MPLA (36 ng/gbw) and anti-CXCR2 or its isotype control (100 μg/200 μl).
Statistical analysis
Data were plotted and analyzed using GraphPad Prism software (La Jolla, CA, USA). Statistical analyses for PMN recruitment, chemokine levels, and bacterial clearance were done using the Mann-Whitney test. Survival curves were analyzed using log rank analyses.
RESULTS
Previous studies from this laboratory demonstrated that CD14−/− mice are resistant to several different clinical isolates of E. coli [13]; protection from infection and accelerated bacterial clearance is associated with an early recruitment of neutrophils to the site of infection (PC) in CD14−/− but not normal mice [12]. Accordingly, the studies described here were initiated to determine the mechanism responsible for this early PMN recruitment in CD14−/− mice and its role in protecting CD14−/− mice to infection by clinical isolates of E. coli. In addition, studies to improve survival of normal mice to severe sepsis by inducing early neutrophil recruitment were undertaken.
PMNs play a critical role in the resistance of CD14−/− mice to severe infection with clinical isolates of E. coli
To characterize neutrophil recruitment in WT and CD14−/− mice infected with clinical isolates of E. coli, differential analysis of the cellular infiltrate in the PC of normal and CD14−/− mice was determined after i.p. injection with either of the two clinical isolates of E. coli.
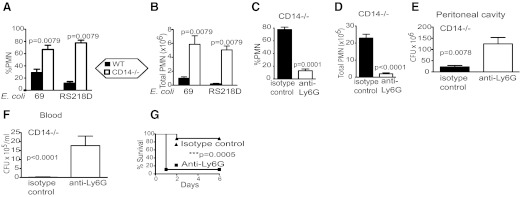
Consistent with our previous observations using a laboratory strain of E. coli [12], within 3 h, there was a dramatic increase in the percentage of white blood cells, which were PMNs in the PC of CD14−/− mice (67–78%) compared with normal mice (11–29%) using either of the two clinical isolates, E. coli 69 and E. coli RS218D (Fig. 1A), respectively. Similarly, comparison of the total number of PMNs recruited to the PC showed five- to 25-fold more PMNs were recruited in CD14−/− mice compared with WT mice in response to the two isolates (Fig. 1B). As a primary role of PMN is to clear bacteria, we examined whether this increased PMN infiltration was responsible for the observed, enhanced clearance of E. coli in CD14−/− mice, which were depleted of PMNs (Fig. 1C and D), by pretreatment with a mAb to the neutrophil-specific Ly6G antigen [16] and after 18 h, injected (i.p.) with a lethal dose of E. coli. Mice pretreated with the anti-Ly6G mAb showed reduced clearance of E. coli in the PC and in blood compared with mice pretreated with the isotype control (Fig. 1E and F). CD14−/− mice pretreated with anti-Ly6G showed no resistance to the E. coli, whereas nearly all of the mice pretreated with an isotype control survived (Fig. 1G).
Figure 1. PMNs are required for bacterial clearance and increased survival in CD14−/− mice in response to E. coli.
(A and B) PMN recruitment in response to two clinical isolates, E. coli 69 and RS218D. Mice [CD14−/− and WT (both C57Bl6), four to five/group] were injected (i.p.) with E. coli isolate 69 (1.9×106 cfu/gbw) or RS218D (2.5×106 cfu/gbw), and after 3 h, mice were euthanized, and the percent PMN in the PC and total PMN count were determined. (C–E) PMN recruitment and bacterial clearance: PMN-depleting antibody, anti-Ly6G (50 μg), was injected 18 h prior to injection of bacteria E. coli RS218D (4.7×106 cfu/gbw) in CD14−/− mice (C57Bl6CD14−/−, nine/group). PMN recruitment to the PC was determined by differential staining of cytospin smears. After 4 h, the percent PMN and total neutrophils recruited were determined. (E and F) The bacteria in the PLF and blood were quantified by viable counts. (G) Survival test: PMN-depleting antibody, anti-Ly6G (50 μg), was injected 18 h prior to injection of E. coli RS218D (3.9×106 cfu/gbw, i.p.) in CD14−/− mice (C57Bl6CD14−/−, nine/group). Survival was monitored over 6 days.
Absence of CD14 greatly reduces the amount of the PMN-attracting chemokines—MIP-2 and KC—in blood but not in the PC
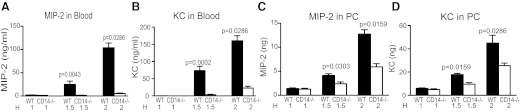
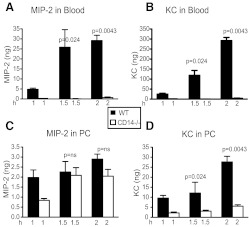
As recruitment of PMNs to a site of infection is the result of responses to a chemokine gradient [18, 19], the concentrations of the two major chemoattractants for PMN—MIP-2 and KC—were determined to gain a better understanding of the mechanism for enhanced recruitment of PMNs to the PC of CD14−/− mice. The levels of the chemokines (MIP-2 and KC) were determined in the blood and PC of normal and CD14−/− mice at various times (1, 1.5, and 2 h) after infection and prior to the 3-h time-point, where large numbers of infiltrating PMNs were observed. Significant amounts of MIP-2 and KC were found in the peripheral blood and PC (Fig. 2) of normal mice within 2 h after i.p. injection with E. coli. Strikingly, in the absence of CD14, there were dramatically lower levels of MIP-2 and KC in the peripheral blood (Fig. 2A and B); this difference was most prominent at the 1.5- and 2-h time-points. Indeed, at 2 h, CD14−/− mice had only 4% and 11%, respectively, of the amount of MIP-2 and KC observed in the blood of normal mice (P=<0.001, Mann Whitney).
Figure 2. Chemokine expression induced in WT and CD14−/− mice in response to live E. coli.
Mice [WT (C57Bl6), four to five/group, and CD14−/− (C57Bl6CD14−/−), four to six/group] were injected (i.p.) with 2–3.5 × 106 cfu/gbw E. coli RS218D, and at 1, 1.5, and 2 h (H) time-points, PLF fluid and blood were recovered, as described in Fig. 1. The levels of the PMN attracting chemokines MIP-2 and KC in the plasma (A and B) and PLF (C and D) were determined by ELISA. Bars, which appear to be absent, have amounts of chemokines close to zero.
In contrast to what was observed in the peripheral blood, the levels of both chemokines in the PC were less-markedly reduced in CD14−/− mice compared with normal mice (Fig. 2C and D), although the differences remained significant. At 2 h, CD14−/− mice had 47% and 57%, respectively, of the amount of MIP-2 and KC observed in the PC of normal mice. This difference in the effect of CD14 on the levels of MIP-2 and KC in peripheral blood versus the PC was consistently observed over many experiments.
Thus, although at early time-points (1 h), the chemokine concentrations in the PC of both strains of mice (WT, CD14−/−) are similar or higher than that in blood, by 1.5 h, the chemokines in the blood of normal mice are significantly higher (**P=0.0079 for MIP2 and KC) than in the PC, as the levels of blood chemokines increase dramatically. Indeed, by 2 h, the ratio of the levels of MIP-2 in the PC to that in blood, (MIP-2)PC/(MIP-2)Bl, was 0.11 in normal mice, whereas in CD14−/− mice, the ratio was 1.34. Similarly, by 2 h, the (KC)PC/(KC)Bl ratio was 0.23 in normal mice and 1.44 in CD14−/− mice. Thus, after infection, normal mice have levels of blood chemokines that greatly exceed the levels in the PC, whereas CD14−/− mice have chemokine levels in the PC that exceed those in blood.
These studies show that chemokine induction by E. coli in normal mice is primarily through a CD14-dependent pathway, although this dependence on CD14 is more striking in blood than in the PC.
Resident peritoneal cells from CD14−/− B6 mice produce chemokines in response to E. coli in vitro
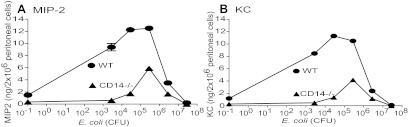
To determine whether resident peritoneal cells might be the source of the chemokines induced in vivo in the PC of CD14−/− mice, the levels of chemokines produced by resident peritoneal cells from WT and CD14−/− mice were compared at increasing concentrations of E. coli RS218D (Fig. 3). At concentrations of 0.028 bacteria/peritoneal cell or less, CD14−/− cells produced negligible amounts of chemokines as compared with the WT cells. However, at concentrations of 0.28 and 2.8 bacteria/peritoneal cell, the induction of MIP-2 (Fig. 3A) and KC (Fig. 3B) by CD14−/− cells was ∼50% that of the WT levels comparable with the ratios observed in vivo in the PC of the two strains of mice. At higher concentrations of 28 bacteria/cell, there was very little chemokine produced by WT and CD14−/− cells.
Figure 3. Induction of chemokines, MIP-2 and KC, by peritoneal cells in vitro in response to E. coli RS218D.
Resident peritoneal cells were isolated from WT and CD14−/− mice and treated with increasing concentrations of E. coli RS218D. After 2.5 h, cell supernatants were collected and analyzed for MIP-2 (A) and KC (B) by ELISA. When error bars are not seen, they fall within the symbol.
LPS, like E. coli, induces PMN recruiting chemokines in the PC of normal and CD14−/− mice but leads to PMN recruitment only to the PC of CD14−/− mice
We observed previously that injection (i.p.) of LPS, a major bacterial component and ligand for the CD14/TLR4 complex, also induced an early and enhanced recruitment of PMN to the PC of CD14−/− but not normal mice [12]. Thus, we sought to determine whether normal and CD14−/− mice injected (i.p.) with LPS showed a pattern of chemokine induction similar to that of live E. coli. Early PMN recruitment was confirmed in these studies at several different time-points (data not shown), while at the same time, the chemokine profiles in the blood and PC were determined. Here again, we observed a striking reduction (>95%) in the levels of the MIP-2 and KC chemokines in the blood of CD14−/− mice compared with normal mice (Fig. 4A and B); in contrast, the levels of these chemokines in the PC were only partially reduced in CD14−/− mice compared with normal mice (Fig. 4C and D). However, it should be noted that the degree of CD14-dependent and independent responses in the PC appears to differ for E. coli versus LPS (Figs. 2 and 4), suggesting that other bacterial components, in addition to LPS, may be driving the CD14-dependent and independent chemokine responses.
Figure 4. Chemokine expression induced in WT and CD14−/− mice in response to LPS.
LPS (from E. coli O111, 0.5 μg/gbw) was injected (i.p.) into mice [WT (C57Bl6, four to five/group) and CD14−/− (C57Bl6CD14−/−, eight to 16/group)], and at 1, 1.5, and 2 h time-points, PLF and blood were collected. Chemokines were analyzed as described in Fig. 3. Bars, which appear to be absent, have amounts of chemokines close to zero.
MPLA induces PMN recruiting chemokines in the PC of normal mice
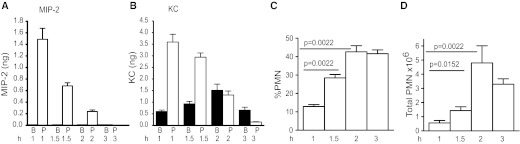
The above observations suggest that the induction of chemokines in the PC, in the absence of induction of excessive chemokines in the blood, results in neutrophil recruitment in response to severe infection with E. coli. We had observed previously that MPLA, unlike LPS or E. coli, is able to induce PMN recruitment even in normal mice when injected i.p [12]. To examine why MPLA but not LPS was able to induce PMN recruitment to the PC of normal mice, the chemokine levels in the PC and blood of normal mice were analyzed at various times after i.p. injection of MPLA. Normal mice (C57BL6) showed a rapid increase (1 h) in the levels of KC and MIP-2 in the PC with very little or none detectable in the blood (Fig. 5A and B). Even at 1.5–2 h for MIP2 and 1.5 h for KC, where we had previously observed significantly higher levels of these chemokines in the blood than in the PC of normal mice injected i.p. with LPS or E. coli (Figs. 2 and 4), there were substantially more chemokines in the PC than in blood when MPLA was used (Fig. 5A and B). This early induction of chemokines in the PC resulted in a significant increase in the percentage of neutrophils in the PC within 2 h (Fig. 5C, P=0.0022). The total number of neutrophils recruited also increased significantly over time (Fig. 5D). The presence of PMN in the PC of normal mice at these early time-points was a result of CXCR2 chemokines, as normal mice pretreated with antibodies to the CXCR2 receptor showed greatly diminished PMN infiltration into the PC and an inability to clear E. coli (data not shown).
Figure 5. PMN recruitment and chemokine profiles induced by MPLA.
MPLA (36 ng/gbw) was injected (i.p.) into mice [WT (C57Bl6), six/group], and at 1, 1.5, 2, and 3 h time-points, chemokine profiles (A and B) and PMN recruitment (C and D) were determined. Bars, which appear absent, have amounts of chemokines close to zero. B, Blood; P, PC.
Induction of chemokines in the PC of normal mice by MPLA prevents bacterial dissemination and death from a localized E. coli infection
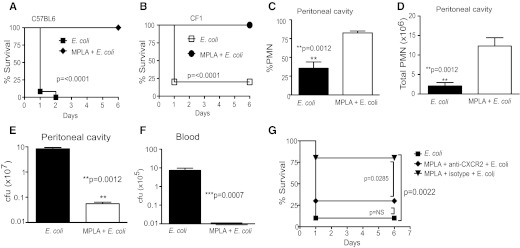
To examine whether this early and rapid induction of PMN recruitment by MPLA in normal mice could protect them from E. coli infection, normal WT mice (both outbred CF1 and inbred C57BL6) were pretreated with MPLA and 2.5 h later, injected (i.p.) with a lethal dose of E. coli. Pretreatment with MPLA offered complete protection from E. coli infection (Fig. 6A and B) and resulted in enhanced recruitment of PMNs (Fig. 6C and D) and rapid clearance of the organism (Fig. 6E and F). Here, we tested the importance of CXCR2 chemokines in the effects of MPLA on infection in normal mice by blocking the CXCR2 receptor. These experiments showed that the protective effects of MPLA were reduced significantly when mice were treated with anti-CXCR2 prior to E. coli infection (Fig. 6G).
Figure 6. MPLA protects against E. coli-induced severe sepsis.
(A and B) WT mice injected with MPLA show survival of a lethal dose of E. coli RS218D. WT mice (CF-1, 15/group, and C57Bl6, 12/group) were injected with E. coli (2.6–3.4×106 cfu/gbw, i.p.), with or without prior injection of MPLA (36 ng/gbw, i.p., 2 h before E. coli). Survival was monitored over 6 days. (C–F) Effects of MPLA on PMN recruitment and bacterial clearance in WT mice injected with E. coli. Mice (C57Bl6, eight to 10/group) were injected i.p. with E. coli RS218D (3.4×106 cfu/gbw), with or without prior injection of MPLA (2 h before E. coli) and PMN recruitment (C and D), and bacterial clearance in the PC (E) and blood (F) was determined 3 h after bacterial injection, as described in Fig. 1. (G) MPLA-induced protection can be reversed by treatment with anti-CXCR2. WT mice [C57Bl6 mice (10/group)] were injected with MPLA (36 ng/gbw) and anti-CXCR2 antibodies (100 μg/mouse) in a single injection; 2.5 h later, mice were injected with E. coli RS218D (3.1×106 cfu/gbw). Controls (10/group) were injected with E. coli RS218D alone or with MPLA and an isotype control antibody, followed 2.5 h later by E. coli RS218D. Survival was monitored over 6 days.
DISCUSSION
Previous studies from this laboratory showed that CD14−/− mice were resistant to lethal doses of a laboratory strain and several clinical isolates of E. coli, injected i.p. [11–13]. This resistance was initially attributed to the tenfold-lower levels of the proinflammatory cytokines TNF and IL-6, induced in CD14−/− mice compared with normal mice [13]. However, as CD14−/− mice were not resistant to a lethal dose of encapsulated (K1) E. coli, in spite of making only low levels of cytokines [13], we reasoned that the resistance of CD14−/− mice to nonencapsulated E. coli might not be solely a result of their low proinflammatory response but might also be related directly to their ability to clear the bacteria. CD14−/− mice had dramatically enhanced clearance of E. coli, both at the site of injection (PC) as well as in blood compared with normal mice [13], which correlates with enhanced neutrophil recruitment to the site of infection.
The studies described here provide an explanation for the enhanced neutrophil recruitment in CD14−/− mice. They suggest that there are two pathways for the induction of chemokines by E. coli, which operate in normal mice: one that is CD14-dependent and induces high chemokine levels primarily in blood with much lower levels induced in the PC and one that is independent of CD14 and induces chemokines in the PC with significantly lower levels in the blood. The CD14-independent pathway leads to rapid PMN infiltration and clearance of the E. coli, which is prevented in normal mice by the induction of high blood chemokines through the CD14-dependent pathway.
Our studies also show that the CD14-independent pathway for chemokine production is in part a result of chemokines produced locally in the PC by the resident peritoneal cells, as MIP2 and KC were produced by CD14−/− resident peritoneal cells after in vitro stimulation with live E. coli (Fig. 3).
Previous studies by others have also suggested the existence of two pathways of chemokine induction [19]: one systemic and one local, which differed in their ability to be induced by thioglycollate. Furthermore, recent reports indicate the existence of a CD14-independent response governing neutrophil recruitment in the liver in response to LPS [20].
The differences in the ability of normal and CD14−/− mice to recruit neutrophils are observed not only for E. coli but also for LPS and are paralleled by the similarity in their chemokine profiles in the blood (high) and PC (low) for normal mice and the opposite distribution for CD14−/− mice. It should also be noted that most experiments described were also done in CD14−/− mice bred >10 generations on Balb/c and C3H/HeN backgrounds; no differences were found, indicating that the genetic background of these strains does not play a role in our conclusions.
In contrast to results with E. coli and LPS, MPLA induces neutrophil recruitment not only in CD14−/− mice but also in normal mice. This appears to be a result of the induction of a significant amount of chemokines in the PC of normal mice without the massive increase in blood chemokines observed with E. coli or LPS. More importantly, the ability of MPLA-induced chemokines to promote neutrophil recruitment and bacterial clearance in normal mice leads to survival after infection with E. coli at doses that normally cause >90% lethality. This protective effect of MPLA is mediated by CXCR2 ligands, as the effect is lost if mice are treated with anti-CXCR2 antibodies (Fig. 6G). Similarly, recent studies from our lab confirmed the importance of the MIP2/KC receptor (CXCR2) in PMN recruitment and bacterial clearance, as antibodies to CXCR2 abrogated the protection seen in CD14−/− mice infected with E. coli (data not shown), resulting in an inability to recruit PMN and to clear the bacteria.
There are several possible explanations for the observation that normal mice, expressing CD14, fail to show PMN recruitment to the PC after E. coli infection. It may be that the induction of high levels of chemokines in the blood relative to the PC creates a chemokine gradient that is not conducive to PMN migration to the PC. Alternatively, as has been suggested previously, the down-regulation of the CXCR2 receptor makes the blood PMN-unresponsive to chemokines released in the PC [21]. Ligand-induced receptor endocytosis induced by high levels of ligand has been reported previously for the CXCR2 receptor [22]. Nonetheless, we attribute the high levels of blood chemokines in normal mice to the gradual systemic dissemination of the organism from the localized site of injection and activation of the CD14-dependent pathway for chemokine induction. Interestingly, in the absence of CD14, this CD14-dependent pathway of chemokine induction is eliminated, with only a relatively small effect on the CD14-independent induction of MIP-2 and KC in the PC of CD14−/− mice.
Several other studies support our observation of enhanced neutrophil infiltration, reduced bacterial load, and protection from bacterial infection or sepsis in CD14−/− mice [23–25], although the mechanisms used are distinct from those described here or were not clearly discernable.
Nevertheless, it should be noted that our observations do conflict with those of several other investigators, where CD14−/− mice show decreased survival compared with WT mice after bacterial challenge [26, 27]. The differences between their results and ours may be attributable, in most cases, to the use of encapsulated organisms, which are resistant to phagocytosis. Indeed, we have shown previously that CD14−/− mice differ markedly in their resistance to isogenic bacterial strains, which differ only in the presence or absence of a K1 capsule [13]. In the presence of an organism with a K1 capsule, the expression of CD14 and TLR4 may indeed be beneficial where it mediates other mechanisms of resistance [26].
In other cases, differences between our results and those of others are likely attributable to additional virulence factors [28], route of infection [29], or differences in LPS structure [30]. Several studies have confirmed the profound effect that LPS structure has on the ability to trigger responses through CD14-dependent versus CD14-independent pathways [30–32].
The studies described illustrate the importance of rapid neutrophil recruitment to the site of infection for mounting an effective defense against bacterial infection, an observation that is supported by other studies [33, 34]. More importantly, they demonstrate how differential activation of distinct chemokine pathways can have a profound effect on bacterial dissemination and survival of the host. Our observations indicate that the high chemokine levels induced early in the blood in normal mice are critical factors in allowing localized E. coli infections to disseminate rapidly. In the presence of multiple pathways for chemokine activation, one of which results in high chemokine levels in the blood, PMN may be misdirected to an inappropriate site (blood), rather than to the source of infection. In this way, E. coli have developed a clever way in which a small number of organisms can act as a decoy and allow the main force (infectious focus) to evade the brunt of the immune response.
In conclusion, these studies support the existence of a CD14-independent pathway for chemokine induction, which is beneficial in protecting against severe E. coli infections. Moreover, the protection conferred by MPLA in E. coli-induced sepsis confirms the ability to harness this pathway for early neutrophil recruitment, leading to enhanced bacterial clearance and survival to severe E. coli infections in normal mice.
ACKNOWLEDGMENTS
This work was supported by NIH, National Institute of Allergy and Infectious Diseases, R01 AI023859. Support for our core facilities was provided by a grant to the Research Centers in Minority Institutions program to The City College of New York from NIH/National Center for Research Resources, G12RR03060. We thank the medical students from Sophie Davis School of Biomedical Education (Jay Guevarra, Sebastian Rubino, and Zohair Hasan) and Ana Pino and Igor Toporovski (Department of Microbiology and Immunology, Sophie Davis School of Biomedical Education) for excellent technical assistance.
Footnotes
- Bl
- blood
- CD14−/−
- CD14-deficient
- gbw
- gram body weight
- KC
- keratinocyte-derived chemokine
- MPLA
- monophosphoryl lipid A
- PC
- peritoneal cavity
- PLF
- peritoneal lavage fluid
- TSA
- trypticase soy agar
- TSB
- trypticase soy broth
AUTHORSHIP
S.M. designed and performed the studies, interpreted the results, and wrote the manuscript. K.S.K. provided reagents, interpreted the results, and wrote the manuscript. J.S. and S. M. G. designed the study, supervised the work, interpreted the results, and wrote the manuscript.
REFERENCES
- 1. Remick D. G. (2007) Pathophysiology of sepsis. Am. J. Pathol. 170, 1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzgerald K. A., Rowe D. C., Golenbock D. T. (2004) Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 6, 1361–1367 [DOI] [PubMed] [Google Scholar]
- 3. Perera P. Y., Mayadas T. N., Takeuchi O., Akira S., Zaks-Zilberman M., Goyert S. M., Vogel S. N. (2001) CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166, 574–581 [DOI] [PubMed] [Google Scholar]
- 4. Remick D. G., Kunkel R. G., Larrick J. W., Kunkel S. L. (1987) Acute in vivo effects of human recombinant tumor necrosis factor. Lab. Invest. 56, 583–590 [PubMed] [Google Scholar]
- 5. Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D., et al. (1986) Shock and tissue injury induced by recombinant human cachectin. Science 234, 470–474 [DOI] [PubMed] [Google Scholar]
- 6. Beutler B., Milsark I. W., Cerami A. C. (1985) Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229, 869–871 [DOI] [PubMed] [Google Scholar]
- 7. Remick D. G., Bolgos G. R., Siddiqui J., Shin J., Nemzek J. A. (2002) Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17, 463–467 [DOI] [PubMed] [Google Scholar]
- 8. Hotchkiss R. S., Coopersmith C. M., McDunn J. E., Ferguson T. A. (2009) The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 15, 496–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H., Czura C. J., Tracey K. J. (2004) Lipid unites disparate syndromes of sepsis. Nat. Med. 10, 124–125 [DOI] [PubMed] [Google Scholar]
- 10. Xiao H., Siddiqui J., Remick D. G. (2006) Mechanisms of mortality in early and late sepsis. Infect. Immun. 74, 5227–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haziot A., Ferrero E., Kontgen F., Hijiya N., Yamamoto S., Silver J., Stewart C. L., Goyert S. M. (1996) Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4, 407–414 [DOI] [PubMed] [Google Scholar]
- 12. Haziot A., Hijiya N., Gangloff S. C., Silver J., Goyert S. M. (2001) Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J. Immunol. 166, 1075–1078 [DOI] [PubMed] [Google Scholar]
- 13. Metkar S., Awasthi S., Denamur E., Kim K. S., Gangloff S. C., Teichberg S., Haziot A., Silver J., Goyert S. M. (2007) Role of CD14 in responses to clinical isolates of Escherichia coli: effects of K1 capsule expression. Infect. Immun. 75, 5415–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Picard B., Garcia J. S., Gouriou S., Duriez P., Brahimi N., Bingen E., Elion J., Denamur E. (1999) The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67, 546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim K. J., Elliott S. J., Di Cello F., Stins M. F., Kim K. S. (2003) The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 5, 245–252 [DOI] [PubMed] [Google Scholar]
- 16. Daley J. M., Thomay A. A., Connolly M. D., Reichner J. S., Albina J. E. (2008) Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 [DOI] [PubMed] [Google Scholar]
- 17. Ness T. L., Hogaboam C. M., Strieter R. M., Kunkel S. L. (2003) Immunomodulatory role of CXCR2 during experimental septic peritonitis. J. Immunol. 171, 3775–3784 [DOI] [PubMed] [Google Scholar]
- 18. Call D. R., Nemzek J. A., Ebong S. J., Bolgos G. R., Newcomb D. E., Wollenberg G. K., Remick D. G. (2001) Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock 15, 278–284 [DOI] [PubMed] [Google Scholar]
- 19. Call D. R., Nemzek J. A., Ebong S. J., Bolgos G. L., Newcomb D. E., Remick D. G. (2001) Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am. J. Pathol. 158, 715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McAvoy E. F., McDonald B., Parsons S. A., Wong C. H., Landmann R., Kubes P. The role of CD14 in neutrophil recruitment within the liver microcirculation during endotoxemia. J. Immunol. 186, 2592–2601 [DOI] [PubMed] [Google Scholar]
- 21. Rios-Santos F., Alves-Filho J. C., Souto F. O., Spiller F., Freitas A., Lotufo C. M., Soares M. B., Dos Santos R. R., Teixeira M. M., Cunha F. Q. (2007) Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am. J. Respir. Crit. Care Med. 175, 490–497 [DOI] [PubMed] [Google Scholar]
- 22. Rose J. J., Foley J. F., Murphy P. M., Venkatesan S. (2004) On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J. Biol. Chem. 279, 24372–24386 [DOI] [PubMed] [Google Scholar]
- 23. Dessing M. C., Knapp S., Florquin S., de Vos A. F., van der Poll T. (2007) CD14 facilitates invasive respiratory tract infection by Streptococcus pneumoniae. Am. J. Respir. Crit. Care Med. 175, 604–611 [DOI] [PubMed] [Google Scholar]
- 24. Wiersinga W. J., de Vos A. F., Wieland C. W., Leendertse M., Roelofs J. J., van der Poll T. (2008) CD14 impairs host defense against gram-negative sepsis caused by Burkholderia pseudomallei in mice. J. Infect. Dis. 198, 1388–1397 [DOI] [PubMed] [Google Scholar]
- 25. Woltmann A., Gangloff S. C., Bruch H. P., Rietschel E. T., Solbach W., Silver J., Goyert S. M. (1999) Reduced bacterial dissemination and liver injury in CD14-deficient mice following a chronic abscess-forming peritonitis induced by Bacteroides fragilis. Med. Microbiol. Immunol. 187, 149–156 [DOI] [PubMed] [Google Scholar]
- 26. Cross A., Asher L., Seguin M., Yuan L., Kelly N., Hammack C., Sadoff J., Gemski P., Jr. (1995) The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J. Clin. Invest. 96, 676–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woods J. P., Frelinger J. A., Warrack G., Cannon J. G. (1988) Mouse genetic locus Lps influences susceptibility to Neisseria meningitidis infection. Infect. Immun. 56, 1950–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang K. K., Dorner B. G., Merkel U., Ryffel B., Schutt C., Golenbock D., Freeman M. W., Jack R. S. (2002) Neutrophil influx in response to a peritoneal infection with Salmonella is delayed in lipopolysaccharide-binding protein or CD14-deficient mice. J. Immunol. 169, 4475–4480 [DOI] [PubMed] [Google Scholar]
- 29. Knapp S., Wieland C. W., Florquin S., Pantophlet R., Dijkshoorn L., Tshimbalanga N., Akira S., van der Poll T. (2006) Differential roles of CD14 and Toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 173, 122–129 [DOI] [PubMed] [Google Scholar]
- 30. Gangloff S. C., Hijiya N., Haziot A., Goyert S. M. (1999) Lipopolysaccharide structure influences the macrophage response via CD14-independent and CD14-dependent pathways. Clin. Infect. Dis. 28, 491–496 [DOI] [PubMed] [Google Scholar]
- 31. Jiang Z., Georgel P., Du X., Shamel L., Sovath S., Mudd S., Huber M., Kalis C., Keck S., Galanos C., Freudenberg M., Beutler B. (2005) CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6, 565–570 [DOI] [PubMed] [Google Scholar]
- 32. Montminy S. W., Khan N., McGrath S., Walkowicz M. J., Sharp F., Conlon J. E., Fukase K., Kusumoto S., Sweet C., Miyake K., Akira S., Cotter R. J., Goguen J. D., Lien E. (2006) Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 33. Alves-Filho J. C., de Freitas A., Spiller F., Souto F. O., Cunha F. Q. (2008) The role of neutrophils in severe sepsis. Shock 30 (Suppl. 1), 3–9 [DOI] [PubMed] [Google Scholar]
- 34. Alves-Filho J. C., Sonego F., Souto F. O., Freitas , A., Verri W. A., Jr., Auxiliadora-Martins M., Basile-Filho A., McKenzie A. N., Xu D., Cunha F. Q., Liew F. Y. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 16, 708–712 [DOI] [PubMed] [Google Scholar]