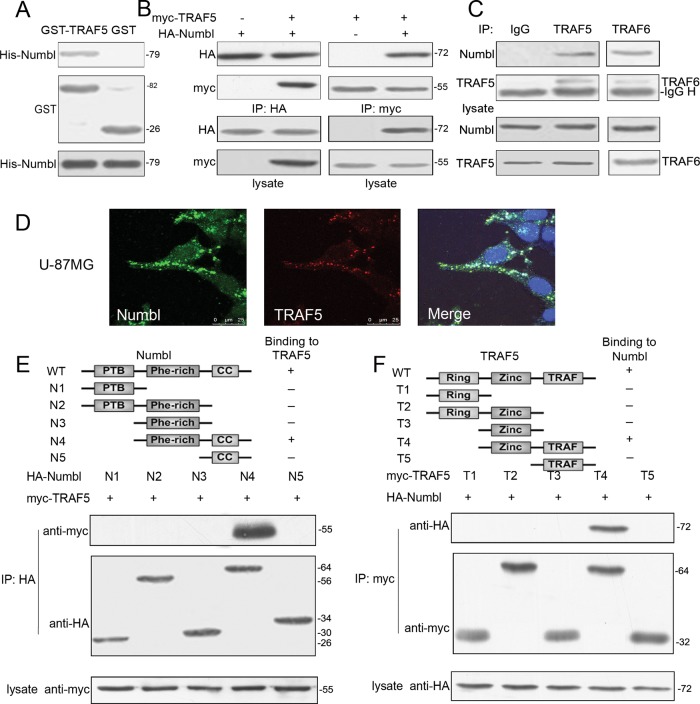
FIGURE 4:
Numbl interacts with TRAF5 in vivo and in vitro. (A) Purified GST-TRAF5 and GST proteins were incubated with His-Numbl in the presence of glutathione beads. The bound proteins were collected and analyzed by Western blotting with anti-His antibody. (B) HA-Numbl and myc-TRAF5 vectors (1.5 μg each) were cotransfected into HEK293T cells. Extracts with equal amount of protein were immunoprecipitated with anti-myc or anti-HA antibody and analyzed by Western blotting. (C) H4 cells were lysed with RIPA buffer and equal amounts of lysates were coimmunoprecipitated with anti-TRAF5 antibody. The normal rabbit immunoglobulin G was used as a negative control, and the TRAF6 antibody was used as a positive control. (D) U-87MG cells were fixed, and immunofluorescence microscopy was performed using the indicated antibodies to show colocalization of endogenous Numbl and TRAF. The nucleus was counterstained with 4′,6-diamidino-2-phenylindole. Scale bar: 25μm. (E) Numbl contains a phosphotyrosine-binding domain (N1), a Phe-rich domain (N3), and a coiled-coil domain (N5). HEK293T cells were transfected with myc-TRAF5 and HA-Numbl mutants (1.5 μg each). Cell lysates were immunoprecipitated with anti-HA antibody and analyzed by Western blotting. (F) TRAF5 contains a finger domain (T1), a zinc finger motif (T3), and a TRAF domain (T5). HEK293T cells were transfected with myc-TRAF5 mutants and HA-Numbl (1.5 μg each). Cell lysates were immunoprecipitated with anti-HA antibody and analyzed by Western blotting.