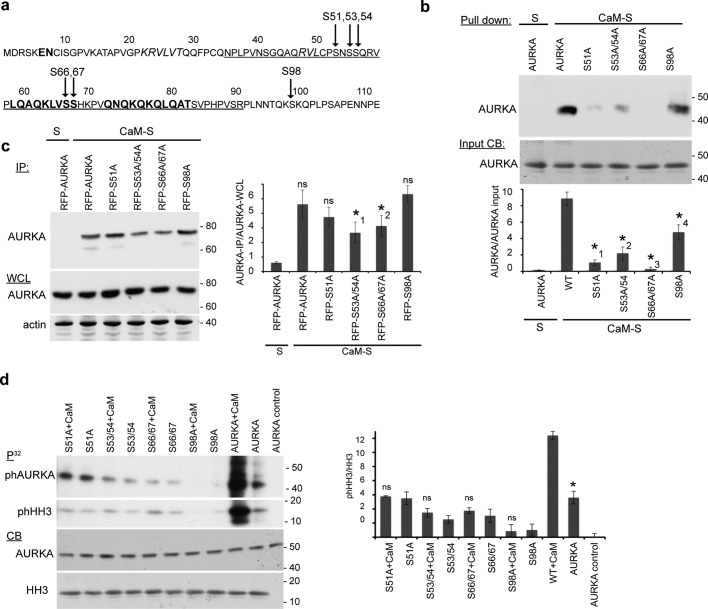
FIGURE 6:
CaM-dependent phosphorylation sites on AURKA (S51, S53, S66/S67, S98) contribute to CaM–AURKA binding. (a) Sequence of disordered AURKA N-terminus (residues 1–127). Predicted β-sheets are shown in italics and α-helices in bold. Arrows indicates Ca2+-dependent AURKA autophosphorylation sites. The strong minimal interaction domain (33–89) with CaM is underlined. (b) Calmodulin–Sepharose (CaM-S) or control Sepharose (S) was used in immunoprecipitation of recombinant His-AURKA and indicated mutated derivatives. CB, Coomassie blue staining of starting material. Graph indicates ratio of AURKA bound to CaM-S, quantified from analysis of three blots; error bars, SE. *1, p = 0.0059. *2, p = 0.011. *3, p = 0.017; *4, p = 0.0074. (c) CaM-S or control S were used for IP from WCL expressing RFP-fused AURKA (WT) and indicated mutated derivatives, followed by Western blotting. Graph indicates ratio of AURKA bound to CaM-S, quantified from analysis of three blots; error bars, SE. *1, p = 0.035; *2, p = 0.045; ns, not significant. (d) In vitro kinase assay using recombinant AURK and mutated derivatives, with the addition of CaM as indicated, assessing AURKA autophosphorylation or phosphorylation of histone H3 (HH3) as substrate. AURKA control lane represents reaction without HH3 or ATP. Graph indicates ratio of phHH3 to total HH3, quantified from analysis of three blots; error bars, SE; *p = 0.015; ns, not significant.