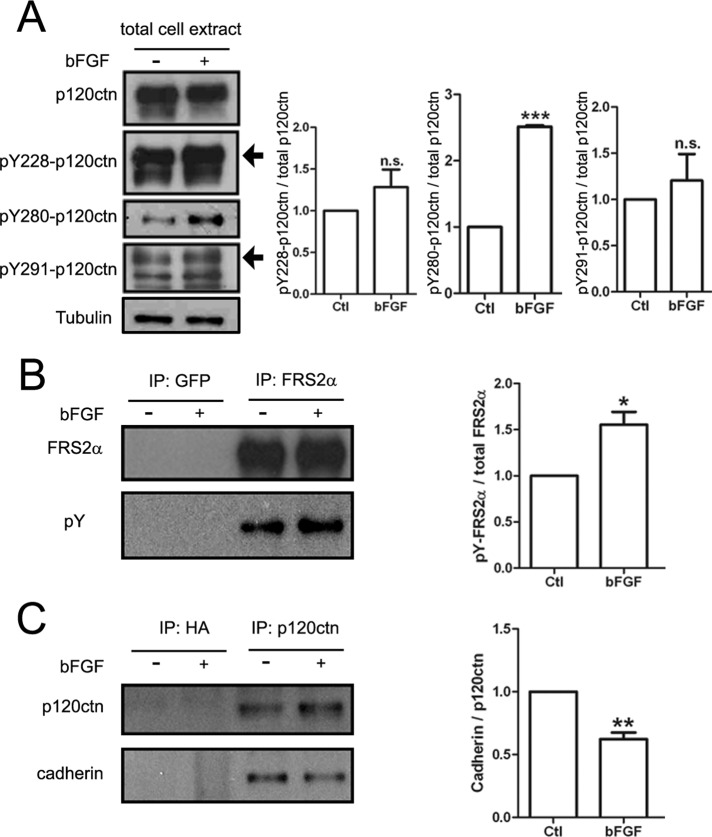
FIGURE 7:
Influence of bFGF on p120ctn's tyrosine phosphorylation and cadherin association. NIH3T3-L1 fibroblasts were serum starved for 16–18 h and then stimulated for 30 min without (–) or with (+) bFGF (200 ng/ml) before preparing total protein extracts. (A) Extracts were immunoblotted with antibodies against p120ctn or p120ctn phosphorylated on three specific tyrosine residues (Y228, Y280, and Y291) and tubulin (loading control). Treatment with bFGF did not alter p120ctn levels in extracts, but it increased the phosphorylation of p120ctn at Y280 robustly and Y228 and Y291 slightly, as shown in the quantification on the right. (B) To confirm that bFGF stimulated the cells in these experiments, we also used a portion of the extracts for immunoprecipitating FRS2α, a downstream target of bFGF signaling. Staining with anti-FRS2α (top) and anti-phosphotyrosine mAb4G10 (pY; bottom) antibodies showed that bFGF stimulation significantly enhanced FRS2α's tyrosine phosphorylation (quantified on the right); a polyclonal GFP antibody used as a negative control did not capture FRS2α. (C) In parallel experiments when p120ctn was immunoprecipitated from extracts (top), under control conditions cadherin (bottom) coprecipitated with p120ctn, but the amount of cadherin associated with p120ctn was reduced by ∼30% after bFGF treatment (quantified on the right); the control anti-hemagglutinin antibody captured neither p120ctn nor cadherin. Ratios of band densities presented in A–C were quantified from three replicates for each set. The ratios of samples from bFGF-treated cells were normalized relative to those from control to obtain the fold change produced by bFGF. Mean and SEM are shown. *p < 0.05, **p < 0.01, ***p < 0.001, compared with control.