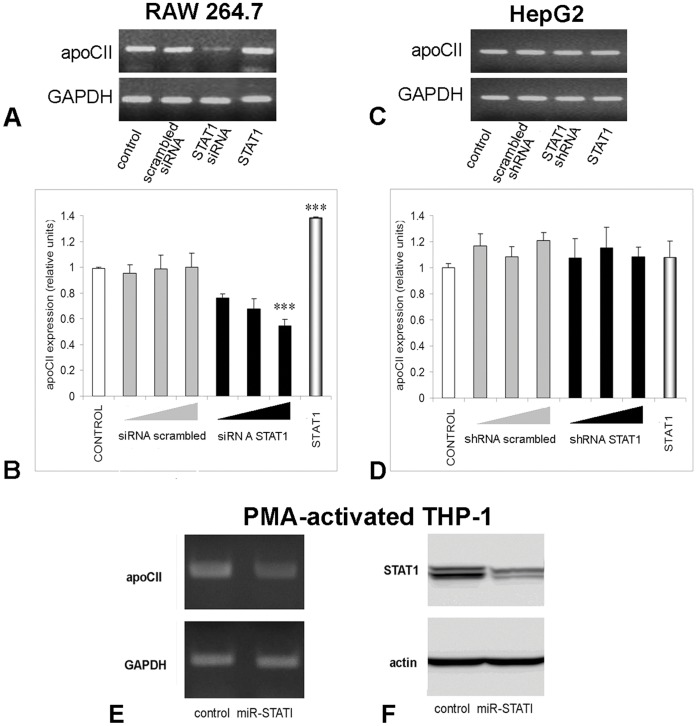
Figure 3. STAT1 positively modulates apoCII expression in macrophages, but not in hepatocytes.
To determine apoCII modulation by STAT1 silencing, RAW 264.7 cells were transfected with siRNA specific for mouse STAT1 (Panel A and B), in HepG2 cells were transfected with STAT1 shRNA producing vector (Panel C and D), and in PMA-activated THP-1 cells were transduced with a lentiviral expression vector for a micro RNA cassette for human STAT1 (Panel E). ApoCII modulation was tested by RT-PCR (A, C and E) and quantified by Real Time PCR (B and D). GAPDH expression was tested to normalize the apoCII expression. The results showed that apoCII expression was strongly inhibited in macrophages (Panel A, lane STAT1 siRNA and Panel E lane miR-STAT1), but not in hepatocytes (Panel C, lane STAT1 shRNA). Real Time PCR experiments showed a dose-dependent inhibition of apoCII by STAT1 siRNA in RAW 264.7 macrophages (for the highest dose, p<0.005), but not in HepG2 cells (Panels B, and D, respectively, black columns). As control, we used scrambled siRNA for RAW.264.7 cells, or scrambled shRNA for HepG2 cells, which did not affect significantly the apoCII expression. STAT1 expression was inhibited more than 50% by transduction of the PMA-activated THP1 with miR-STAT1, as detected by Western blotting (Panel F). To determine apoCII modulation by STAT1 overexpression, RAW 264.7 macrophages and HepG2 cells were transfected with a STAT1 expression vector and subjected to RT-PCR. ApoCII mRNA level was increased in STAT1-overexpressing macrophages (Panel A, lane STAT1), but not in hepatocytes (Panel C lane STAT1, and Panel D last column). Real Time PCR experiments revealed an increase of ∼1.4 folds in apoCII expression in STAT1 overexpressing macrophages (Panels B, last columns; p<0.005).