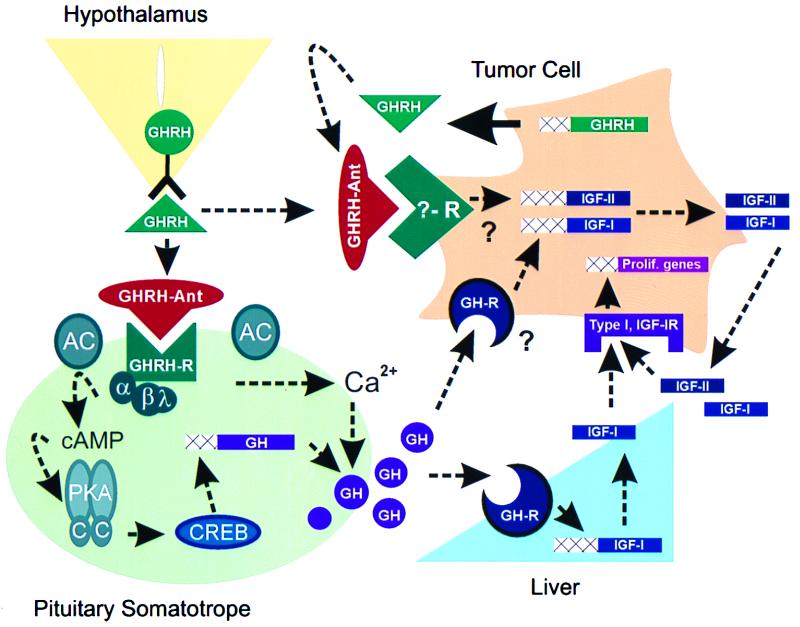
Figure 1.
Potential mechanisms mediating the antitumorigenic actions of GHRH antagonists (GHRH-Ant). GHRH antagonists bind to GHRH receptors (GHRH-R), located on pituitary somatotropes, and block GH synthesis and release. The GHRH receptor is a seven-transmembrane, G-protein-coupled receptor and is a member of the receptor superfamily that includes the VIP and PACAP receptors. Binding of GHRH to its receptor activates the α-subunit (Gs) of the closely associated G-protein complex, thus stimulating membrane bound adenylyl cyclase (AC) and increasing intracellular cAMP concentrations. cAMP binds to and activates the regulatory subunits of PKA, which in turn release catalytic subunits (C) that translocate to the nucleus and phosphorylate the cAMP response element binding protein, CREB. CREB, via direct and indirect mechanisms, stimulates GH gene transcription (3). In addition, GHRH-mediated cAMP-dependent and cAMP-independent pathways cause an influx of extracellular Ca2+, leading to the release of GH secretory vesicles and resulting in a rapid increase in circulating GH concentrations (3). GH stimulates liver IGF-I gene transcription (37) and could directly stimulate tumor IGF-I production. GH-induced increases in IGF-I could activate type I IGF-I receptors located on tumor cells, thereby mediating the transcription of genes important for cell proliferation (5). It is also possible that GHRH antagonists directly bind to and block a yet to be identified receptor that mediates the stimulatory effects of locally produced GHRH on IGF-II production. Locally produced IGF-II can in turn activate cell proliferation by binding to type I IGF-I receptors (5, 12). Dashed arrows indicate pathways suppressed after application of GHRH antagonists. Theoretical pathways are denoted by question marks.