Abstract
AIM: To clarify the strategy for early diagnosis of pancreaticobiliary maljunction (PBM) without biliary dilatation and to pathologically examine gallbladder before cancer develops.
METHODS: The anatomy of the union of the pancreatic and bile ducts was assessed by using endoscopic retrograde cholangiopancreatography (ERCP). Patients with a long common channel in which communication between the pancreatic and bile ducts was maintained even during sphincter contraction were diagnosed as having PBM. Of these, patients in which the maximal diameter of the bile duct was less than 10 mm were diagnosed with PBM without biliary dilatation. The process of diagnosing 54 patients with PBM without biliary dilatation was retrospectively investigated. Histopathological analysis of resected gallbladder specimens from 8 patients with PBM without biliary dilatation or cancer was conducted.
RESULTS: Thirty-six PBM patients without biliary dilatation were diagnosed with gallbladder cancer after showing clinical symptoms such as abdominal or back pain (n = 16) or jaundice (n = 12). Radical surgery for gallbladder cancer was only possible in 11 patients (31%) and only 4 patients (11%) survived for 5 years. Eight patients were suspected as having PBM without biliary dilatation from the finding of gallbladder wall thickening on ultrasound and the diagnosis was confirmed by ERCP and/or magnetic resonance cholangiopancreatography (MRCP). The median age of these 8 patients was younger by a decade than PBM patients with gallbladder cancer. All 8 patients underwent prophylactic cholecystectomy and bile duct cancer has not occurred. Wall thickness and mucosal height of the 8 resected gallbladders were significantly greater than controls, and hyperplastic changes, hypertrophic muscular layer, subserosal fibrosis, and adenomyomatosis were detected in 7 (88%), 5 (63%), 7 (88%) and 5 (63%) patients, respectively. Ki-67 labeling index was high and K-ras mutation was detected in 3 of 6 patients.
CONCLUSION: To detect PBM without biliary dilatation before onset of gallbladder cancer, we should perform MRCP for individuals showing increased gallbladder wall thickness on ultrasound.
Keywords: Pancreaticobiliary maljunction, Pancreatobiliary reflux, Ultrasound, Gallbladder cancer, Endoscopic ultrasonography
INTRODUCTION
Pancreaticobiliary maljunction (PBM) is a congenital anomaly defined as a junction of the pancreatic and bile ducts located outside the duodenal wall, and usually forming a markedly long common channel. As the action of the sphincter of oddi does not have a functional impact on the junction of the pancreatic and bile ducts in PBM cases, PBM causes a continuous reciprocal reflux of pancreatic juice and bile[1,2]. This results in various pathological conditions of the biliary tract and pancreas. Given that the hydropressure in the pancreatic duct is usually greater than that in the bile duct, pancreatic juice frequently flows back into the biliary duct (pancreatobiliary reflux), and this becomes a high risk factor for biliary cancer[3-5]. In a Japanese analysis[6], biliary cancer was associated with 131 (10.6%) of 1239 cases with PBM with biliary dilatation (congenital choledochal cyst); 44 (33.6%) of the 131 cases had cancer of the extrahepatic bile duct and 85 cases (64.9%) had gallbladder cancer. On the other hand, PBM without biliary dilatation was associated with biliary cancer in 147 (37.9%) of 388 cases, and 137 (93.2%) of these cancers were gallbladder cancer.
Most PBM cases detected in childhood are associated with bile duct dilatation, but one third of PBM detected in adults do not show dilatation of the bile duct. Many patients with PBM with biliary dilatation have clinical symptoms due to cholangitis or pancreatitis in childhood, whereas in PBM without biliary dilatation, few patients have symptoms in childhood and they are usually not diagnosed until adulthood[7]. Furthermore, many patients with PBM without biliary dilatation are diagnosed in association with advanced-stage gallbladder cancer, which carries a poor prognosis[2,6,7]. It is necessary to clarify a strategy to diagnose PBM without biliary dilatation early, before cancer occurs. In the present study, we investigated the process for diagnosing patients with PBM without biliary dilatation and examined histopathological findings from the gallbladder of patients with PBM without biliary dilatation before cancer developed.
MATERIALS AND METHODS
Study patients
We studied the anatomy of the union of the pancreatic and bile ducts using endoscopic retrograde cholangiopancreatography (ERCP). Patients with a long common channel in which communication between the pancreatic and bile ducts was maintained in both relaxation and contraction of the sphincter under serial observation during ERCP were diagnosed as having PBM[1,2]. Of these, patients in whom the maximal diameter of the bile duct was less than 10 mm were diagnosed with PBM without biliary dilatation (Figure 1). When the common bile duct was involved with associated gallbladder cancer, diameter of the intact distal portion of the bile duct was measured.
Figure 1.
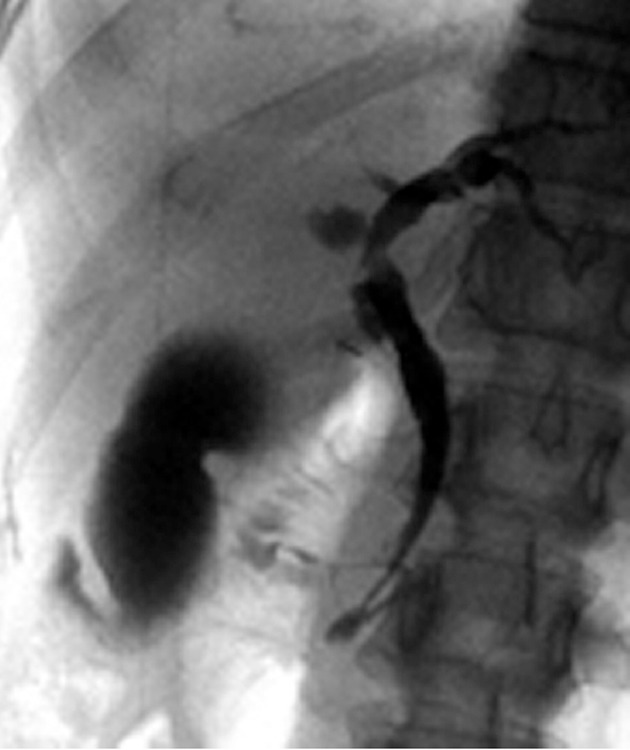
Endoscopic retrograde cholangiopancreatography of a patient with pancreaticobiliary maljunction without biliary dilatation showing a long common channel and deformity with fuzzy irregularity of the gallbladder.
Between January 1975 and December 2010, 104 patients were diagnosed with PBM with ERCP. Endoscopic ultrasonography (EUS) and magnetic resonance cholangiopancreatography (MRCP) were performed from 1990 and 2001, respectively. Of these, 54 patients (12 men and 42 women; median age at initial diagnosis of PBM 56.6 years, range 30-77 years) were diagnosed with PBM without biliary dilatation. The process leading to diagnosis of these patients was retrospectively investigated. This study was approved by the institutional review board.
Histopathological and immunohistochemical examinations
We conducted histopathological analysis of resected gallbladder specimens from 8 patients with PBM without biliary dilatation or cancer. Tissues were fixed in 10% buffered formalin and embedded in paraffin. Serial 3-μm sections were stained with hematoxylin and eosin for evaluation. Thickness of the gallbladder wall and mucosal height of each specimen were measured using a semiautomatic image analyzer. Hyperplastic changes were defined as an increased number of mucosal folds that were longer than normal, irregular, and frequently branched. Immunohistochemistry was performed using antisera for Ki-67 (clone MIB-1: Immunotech SA, Marseille, France) by the avidin-biotin horseradish peroxidase method (Vectastain Elite ABC kit; Vector, Burlingame, CA, United States). Ki-67-positive cells were defined as a cell with brown staining of the nucleus, and Ki-67 labeling index was determined by counting a minimum of 500 cells in the area representing the most homogenous region of positive cells.
Gallbladder epithelium was microdissected from 20-μm formalin-fixed paraffin-embedded sections. Genomic DNA was extracted from the tissues by using Takara Dexpattm (Takara Shuzo, Otsu, Japan). A point mutation of K-ras codon 12 was analyzed by polymerase chain reaction enzyme-linked minisequence assay (Sumitomo Metal Industry, Tokyo, Japan). The precise methodology of this assay is described elsewhere[8].
Ten cases of normal gallbladder that were resected at pancreatoduodenectomy for pancreatic diseases were selected as controls.
Statistical analysis
Statistical analysis was performed using chi-squared analysis or Mann-Whitney’s U test. P values of less than 0.05 were considered statistically significant.
RESULTS
Process of diagnosing PBM without biliary dilatation
Clues associated with a diagnosis of PBM without biliary dilatation were the presence of gallbladder cancer (n = 36), other malignancies (n = 4), chronic pancreatitis (n = 1), intrahepatic stones (n = 1), gallstones (n = 1), gastritis (n = 1), previous cholecystectomy (n = 2), and gallbladder wall thickening (more than 3 mm) on ultrasound (US; n = 8) (Figure 2). Thirty-one patients with gallbladder cancer had clinical symptoms such as abdominal or back pain (n = 16), jaundice (n = 12), and abdominal discomfort (n = 3), and the other 5 gallbladder cancers were detected using screening tests such as US (n = 4) and fluorode-oxyglucose positron emission tomography (n = 1). Radical surgery was possible in only 11 (31%) of 36 cases of gallbladder cancer and only 4 patients (11%) survived for 5 years. Three PBM patients without biliary dilatation associated with benign diseases (chronic pancreatitis, gallstones and gastritis) were diagnosed before 1985, and they were lost to follow up without surgery.
Figure 2.
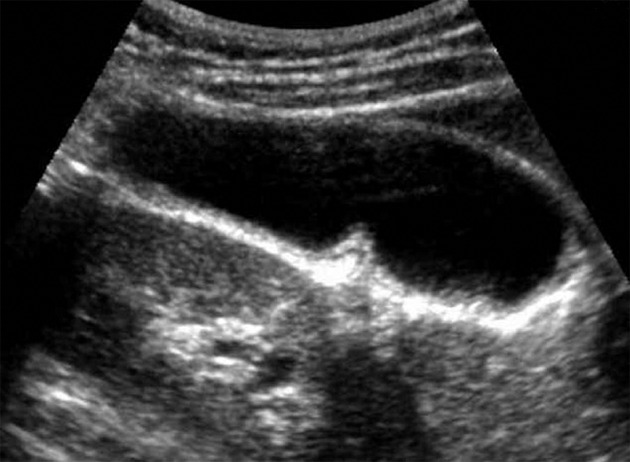
Abdominal ultrasound of a patient with pancreaticobiliary maljunction without biliary dilatation showing uniform smooth thickness of the gallbladder wall.
PBM patients without biliary dilatation diagnosed from gallbladder wall thickening on US
PBM without biliary dilatation was suspected in 8 patients based on finding of gallbladder wall thickening on US. Abdominal US was performed for symptoms such as abdominal pain in 5 patients to screen for associated diseases. Following US, computed tomography (n = 8), EUS (n = 5), MRCP (n = 4), and ERCP (n = 8) were done. The median age of patients was 48.2 years (range 30-70 years; 3 men and 5 women), which was significantly younger than that of PBM patients with gallbladder cancer (median age, 58.5 years; P < 0.01). Based on the diagnosis of PBM without biliary dilatation, all patients underwent prophylactic cholecystectomy. Amylase levels in the bile were markedly elevated in all patients. Five patients have been regularly followed, and 3 patients were lost to follow up 1-75 mo after surgery. Bile duct cancer has not occurred in any patient (Table 1). On EUS, thickening of the inner hypoechoic layer and outer hyperechoic layer was detected in 2 patients (Figure 3), and thickening of the inner hypoechoic layer, a middle, more hypoechoic layer, and the outer hyperechoic layer was observed in 3 patients.
Table 1.
Clinical features of 8 pancreaticobiliary maljunction patients without biliary dilatation or cancer
| Case | Age (yr) | Sex | Process leading to ultrasound | Amylase level in bile (IU/L) | Follow-up |
| 1 | 30 | F | Screening for hepatitis A | 528 000 | Follow-up after 236 mo |
| 2 | 55 | F | Screening for chronic hepatitis C | 69 850 | Follow-up after 47 mo |
| 3 | 65 | F | Cholangitis with mild acute pancreatitis | 17 272 | Follow-up after 40 mo |
| 4 | 41 | F | Right hypochondralgia | 117 240 | Follow-up after 12 mo |
| 5 | 70 | F | Screening for diabetes mellitus | 254 900 | Follow-up after 3 mo |
| 6 | 42 | M | Diarrhea | 185 000 | Unknown after 75 mo |
| 7 | 47 | M | Abdominal pain | 300 000 | Unknown after 18 mo |
| 8 | 36 | M | Abdominal discomfort | 672 100 | Unknown after 1 mo |
Figure 3.
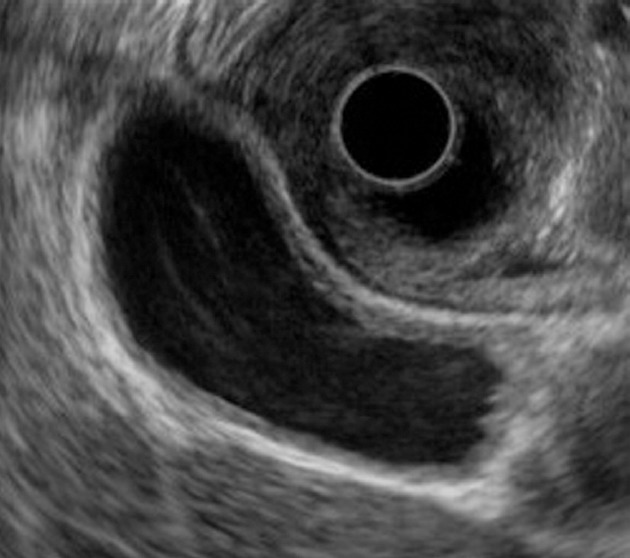
Endoscopic ultrasonography of a patient with pancreaticobiliary maljunction without biliary dilatation showing thickening of the inner hypoechoic layer and the outer hyperechoic layer.
Histopathological findings of the gallbladder of PBM patients without biliary dilatation diagnosed from gallbladder wall thickening on US
On histological findings of the resected gallbladder, the gallbladder wall was more thickened in PBM (7.7 ± 7.0 mm, mean ± SD) than in control cases (2.0 ± 0.5 mm, P < 0.01). Mucosal height was significantly higher in PBM (1.0 ± 0.7 mm) than in control cases (0.5 ± 0.2 mm, P < 0.01). Hyperplastic changes, hypertrophic muscular layer, subserosal fibrosis, and adenomyomatosis were detected in 7 (88%), 5 (63%), 7 (88%) and 5 (63%) of PBM patients, respectively, compared with no control cases (Figure 4). No dysplastic changes were observed. Ki-67 labeling index was higher in PBM (6.0% ± 2.1%) than control cases (1.2% ± 0.3%, P < 0.01). K-ras mutation was detected in 3 (50%) of 6 PBM patients (Table 2). Adenomatous changes were not observed in any patient.
Figure 4.
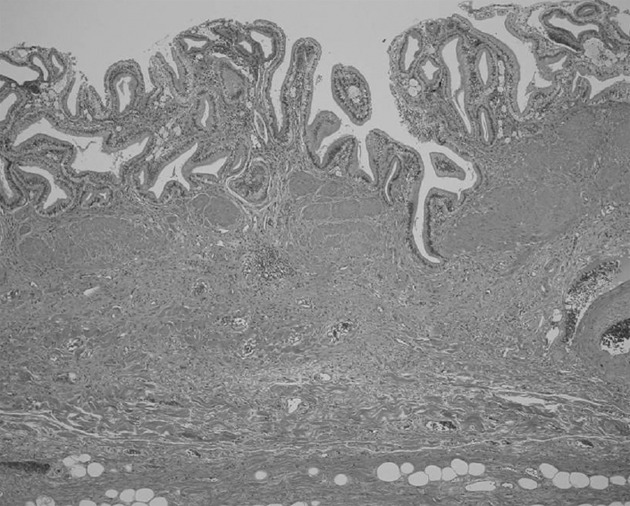
Histological findings of the gallbladder of a patient with pancreaticobiliary maljunction without biliary dilatation showing wall thickness composed of epithelial hyperplasia, hypertrophic muscular layer, and subserosal fibrosis.
Table 2.
Histopathological findings of gallbladders from patients with pancreaticobiliary maljunction without biliary dilatation diagnosed based on gallbladder wall thickening on ultrasound
| Case | Thickness of the wall (mm) | Mucosal height (mm) | Hyperplastic change | Hypertrophic muscular layer | Subserosal fibrosis | Adenomyomatosis | Gallstone | Ki-67 labeling index (%) | K-ras mutation |
| 1 | 7 | 2.4 | +a | - | + | - | - | 8 | GAT (3+) |
| 2 | 6 | 1.8 | + | - | + | + | - | 5 | NA |
| 3 | 3 | 0.6 | - | - | - | - | + | 6 | - |
| 4 | 5 | 2.2 | + | + | + | + | - | 4 | - |
| 5 | 5 | 1.7 | + | + | + | + | - | 5 | NA |
| 6 | 8 | 1.9 | + | + | + | + | - | 8 | AGT (1+) |
| 7 | 6 | 1.4 | + | + | + | - | + | 9 | AGT (2+) |
| 8 | 25 | 0.9 | + | + | + | + | - | 3 | - |
| PBM, %, | 7.7 ± 7.0b | 1.06 ± 0.7b | 88 (7/8)b | 63 (5/8)b | 88 (7/8)b | 63 (5/8)b | 25 (2/8) | 6.0 ± 2.1b | 50 (3/6)a |
| mean ± SD | |||||||||
| Controls, %, | 2.0 ± 0.5 | 0.58 ± 0.24 | 0 (0/10) | 0 (0/10) | 0 (0/10) | 0 (0/10) | 10 (1/10) | 1.2 ± 0.3 | 0 (0/10) |
| (n = 10), | |||||||||
| mean ± SD |
PBM: Pancreaticobiliary maljunction; NA: Not amplified.
P < 0.05,
P < 0.01 vs controls.
DISCUSSION
Because PBM patients have a high incidence of biliary cancer due to pancreatobiliary reflux, once PBM is diagnosed, prophylactic biliary surgery is recommended before the onset of malignant change[2]. In particular, PBM without biliary dilatation is frequently associated with gallbladder cancer. However, PBM cases without biliary dilatation rarely evoke symptoms, and most patients are not diagnosed until the onset of advanced-stage gallbladder cancer[2,6,7].
In this series, 36 (67%) of 54 PBM patients without biliary dilatation were diagnosed based on associated gallbladder cancer. Most gallbladder cancers were in an advanced stage, and resection was possible in only 11 patients (31%). Since a paper[9] published in 1991 reported that gallbladder wall thickening was sometimes observed in PBM cases without biliary dilatation, we have prospectively checked for PBM after finding gallbladder wall thickening on US. This additional investigation using MRCP and ERCP allowed us to identify 8 patients with PBM without biliary dilatation before the onset of gallbladder cancer. All 8 patients underwent prophylactic cholecystectomy, and bile duct cancer has not occurred in any patient. The median age of the 8 patients was about 10 years younger than PBM patients with associated gallbladder cancer. The age of PBM patients with gallbladder cancer at the time of diagnosis peaked in the 50s, and was younger by a decade than that of gallbladder cancer patients without PBM[10]. Chronic injury to the epithelium of bile duct by pancreatic juice induces severe inflammation and malignant changes in PBM patients; therefore, age can be a major determinant of risk. To eliminate the risk of gallbladder cancer, we must detect PBM without biliary dilatation early in adults.
Epithelial hyperplasia of the gallbladder induced by longstanding continuous stasis of the bile intermingled with refluxed pancreatic juice has been reported to be one of the characteristic pathological changes in PBM patients[11-13]. The incidence of epithelial hyperplasia of the gallbladder of PBM patients without biliary dilatation was reported to be 72%[11] to 91%[12]. Tanno et al[12] reported that the Ki-67 labeling index of epithelial hyperplasia of PBM patients was elevated to 6.1% ± 1.5% and K-ras mutation was detected in 2 (13%) of 15 patients. Histopathological findings of our 8 cases were similar to the above findings. Considering that increased cell proliferation is linked to the development of cancer by means of tumor promotion and an increased rate of random mutations, gallbladder epithelium of PBM patients can be considered to represent a premalignant lesion.
Interestingly, hypertrophic muscular layer, subserosal fibrosis, and adenomyomatosis were detected in 63%, 88% and 63%, respectively. Sugai et al[14] reported that subserosal fibrosis with chronic inflammation was detected in 33% of gallbladders of 27 PBM patients, and Tsuchida et al[11] found K-ras mutations in 5 (50%) of 10 gallbladders of PBM without biliary dilatation. Long-standing continuous stimulation with bile containing pancreatic juice may induce these three changes as well as epithelial hyperplasia.
EUS shows the normal gallbladder wall to be a two-layered structure consisting of an inner hypoechoic layer composed of the mucosa and the muscular layer, and an outer hyperechoic layer composed of the subserosal layer and the serosa[15]. On EUS, the gallbladder wall of patients with PBM appeared as two thickened layers showing epithelial hyperplasia and subserosal fibrosis or three thickened layers containing a middle, more hypoechoic, layer showing a hypertrophic muscular layer.
Although ERCP is the gold standard for diagnosis of PBM, MRCP has recently become a common non-invasive method for obtaining quality images of the pancreaticobiliary tract. MRCP can diagnose many PBM cases based on findings of an anomalous union between the common bile duct and the pancreatic duct, although some atypical PBM cases with relatively short common channel cannot be diagnosed only by MRCP, and should be confirmed by ERCP[16]. To detect patients with PBM without biliary dilatation early requires that certain patients undergo MRCP before gallbladder cancer occurs. Gallbladder wall thickness on US during medical checkups may serve as an indication for MRCP and EUS before ERCP for suspected PBM without biliary dilatation. Once PBM is diagnosed in patients with increased gallbladder wall thickness, prophylactic biliary surgery is recommended before the onset of malignant change.
In conclusion, hyperplastic gallbladder mucosa of PBM patients represents a premalignant lesion. To detect PBM without biliary dilatation before onset of gallbladder cancer, we should perform MRCP for individuals showing gallbladder wall thickness on US.
COMMENTS
Background
Pancreaticobiliary maljunction (PBM) causes a continuous reciprocal reflux of pancreatic juice and bile. PBM cases without biliary dilatation rarely evoke symptoms, and most patients are not diagnosed until the onset of advanced-stage gallbladder cancer. It is necessary to clarify a strategy to diagnose PBM without biliary dilatation early, before cancer occurs.
Research frontiers
The authors investigated the process for diagnosing patients with PBM without biliary dilatation and examined histopathological findings from the gallbladder of patients with PBM without biliary dilatation before cancer developed.
Innovations and breakthroughs
Thirty-six (66%) out of 54 PBM patients without biliary dilatation were diagnosed with gallbladder cancer. Radical surgery for gallbladder cancer was only possible in 11 patients and only 4 patients survived for 5 years. Eight patients were suspected as having PBM without biliary dilatation from the finding of gallbladder wall thickening on ultrasound and the diagnosis was confirmed by endoscopic retrograde cholangiopancreatography and/or magnetic resonance cholangiopancreatography (MRCP). The median age of these 8 patients was younger by a decade than PBM patients with gallbladder cancer. All 8 patients underwent prophylactic cholecystectomy and bile duct cancer has not occurred. Wall thickness and mucosal height of the 8 resected gallbladders were significantly greater than controls, and hyperplastic changes were detected in 7 (88%) patients. Ki-67 labeling index was high and K-ras mutation was detected in 3 of 6 patients.
Applications
To detect PBM without biliary dilatation before onset of gallbladder cancer, people should perform MRCP for individuals showing increased gallbladder wall thickness on ultrasound.
Peer review
The study has a good-sized patient cohort and involves appropriate methods of analysis. The interpretation and presentation of the results are both appropriate. The findings of this study should contribute in the effective management of patients with PBM.
Footnotes
Peer reviewers: Barjesh Chander Sharma, Professor, Depart-ment of Gastroenterology, G.B. Pant Hospital, Room 203, Academic Block, New Delhi 110002, India; Tedros Bezabeh, National Research Council Institute for Biodiagnostics, 435 Ellice Avenue, Winnipeg R3B 1Y6, Canada; Dr. Jean Louis Frossard, Department of Internal Medicine, Division of Gastroenterology, Rue Micheli du Crest, 1211 Geneva, Switzerland
S- Editor Gou SX L- Editor Logan S E- Editor Li JY
References
- 1.The Japanese study group on Pancreaticobiliary Maljunction. Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 1994;1:219–221. [Google Scholar]
- 2.Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K, Sasaki T. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol. 2009;7:S84–S88. doi: 10.1016/j.cgh.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Csendes A, Kruse A, Funch-Jensen P, Oster MJ, Ornsholt J, Amdrup E. Pressure measurements in the biliary and pancreatic duct systems in controls and in patients with gallstones, previous cholecystectomy, or common bile duct stones. Gastroenterology. 1979;77:1203–1210. [PubMed] [Google Scholar]
- 4.Carr-Locke DL, Gregg JA. Endoscopic manometry of pancreatic and biliary sphincter zones in man. Basal results in healthy volunteers. Dig Dis Sci. 1981;26:7–15. doi: 10.1007/BF01307970. [DOI] [PubMed] [Google Scholar]
- 5.Arendt T, Stoffregen C, Kloehn S, Mönig H, Nizze H, Fölsch UR. Santorini’s duct--risk factor for acute pancreatitis or protective morphologic variant? Experiments in rabbits. Eur J Gastroenterol Hepatol. 1997;9:569–573. doi: 10.1097/00042737-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, Shimada H, Takamatsu H, Miyake H, Todani T. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. 2003;10:345–351. doi: 10.1007/s00534-002-0741-7. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchida A, Aoki T, Ozawa T, Inoue K, Mimuro A, Ikeda T, Nakamura R, Kitamura K, Koyanagi Y. Surgical treatment of pancreaticobiliary maljunction without bile duct dilatation in adult cases. In: Koyanagi Y, Aoki T, editors. Pancreaticobiliary Maljunction. Tokyo: Igaku Tosho; 2002. pp. 331–337. [Google Scholar]
- 8.Kamisawa T, Tsuruta K, Okamoto A, Horiguchi S, Hayashi Y, Yun X, Yamaguchi T, Sasaki T. Frequent and significant K-ras mutation in the pancreas, the bile duct, and the gallbladder in autoimmune pancreatitis. Pancreas. 2009;38:890–895. doi: 10.1097/MPA.0b013e3181b65a1c. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi H. Imaging features of the gallbladder wall in patients with anomalous arrangement of the pancreaticobiliary ductal system. J Jpn Biliary Association. 1991;5:517–525. [Google Scholar]
- 10.Ohta T, Nagakawa T, Ueno K, Maeda K, Ueda N, Kayahara M, Akiyama T, Kanno M, Konishi I, Izumi R. Clinical experience of biliary tract carcinoma associated with anomalous union of the pancreaticobiliary ductal system. Jpn J Surg. 1990;20:36–43. doi: 10.1007/BF02470711. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida A, Itoi T, Endo M, Kitamura K, Mukaide M, Itokawa F, Ozawa T, Aoki T. Pathological features and surgical outcome of pancreaticobiliary maljunction without dilatation of the extrahepatic bile duct. Oncol Rep. 2004;11:269–276. [PubMed] [Google Scholar]
- 12.Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, Ura H, Klein-Szanto AJ, Kohgo Y. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer. 1998;83:267–275. [PubMed] [Google Scholar]
- 13.Yamamoto M, Nakajo S, Tahara E, Ito M, Taniyama K, Shimamoto F, Miyoshi N, Hayashi Y, Akiyama H, Nakai S. Mucosal changes of the gallbladder in anomalous union with the pancreatico-biliary duct system. Pathol Res Pract. 1991;187:241–246. doi: 10.1016/S0344-0338(11)80778-8. [DOI] [PubMed] [Google Scholar]
- 14.Sugai M, Ishido K, Endoh M, Hada R, Munakata H. Sonographic demonstration of wall thickness of the gallbladder in pediatric patients with pancreatico-biliary maljunction. J Hepatobiliary Pancreat Sci. 2010;17:345–348. doi: 10.1007/s00534-009-0252-x. [DOI] [PubMed] [Google Scholar]
- 15.Fujita N, Noda Y, Kobayashi G, Yoga A. Analysis of the layer structure of the gallbladder wall delineated by endoscopic ultrasonography. Digest Endosc. 1995;7:353–356. [Google Scholar]
- 16.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129–133. doi: 10.1007/s00261-006-9005-3. [DOI] [PubMed] [Google Scholar]


