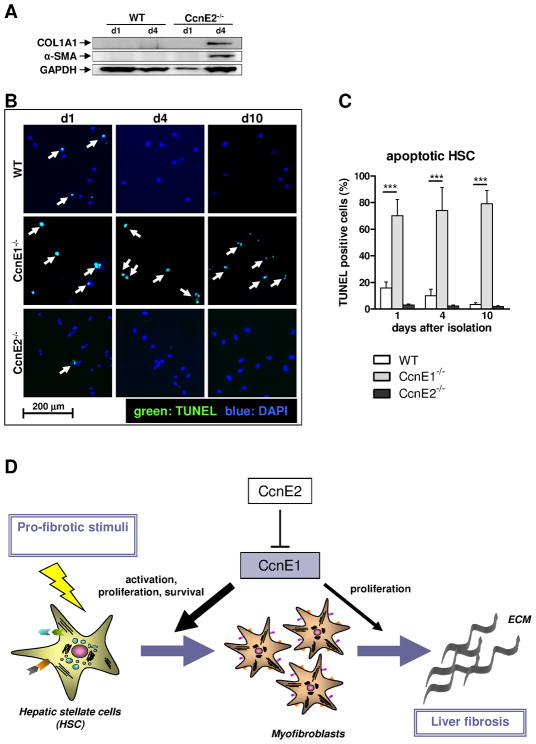
Figure 7. CcnE1 expression is crucial for HSC survival and its transdifferentiation into myofibroblasts.
(A) Protein expression analysis of collagen 1 and α-SMA was determined in WT- and CcnE2−/− HSC at day 1 (d1) and day 4 (d4) after isolation. Expression of GAPDH was used as loading control. (B) TUNEL analysis of ex vivo isolated primary HSC. TUNEL-positive, apoptotic cells are stained in green and highlighted by arrows. Nuclei of total cells were stained with DAPI and are shown in blue. (C) Quantification of apoptotic cells shown in (D). ***: p<0.001. (D) Proposed model to explain the pro-fibrogenic function of CcnE1. CcnE1 is negatively regulated by CcnE2. After pro-fibrotic stimulation e.g. toxic liver injury, CcnE1 is up-regulated at an early stage and drives the initial cell cycle activation of hitherto quiescent HSC. HSC activation results in transdifferentiation into myofibroblast, which produce extracellular matrix (ECM) and are essential for fibrogenesis. Maximal CcnE1 expression in HSC occurs prior to transdifferentiation. Thus CcnE1 is essential for early HSC activation and survival, but presumably less important for proliferation of differentiated myofibroblasts.