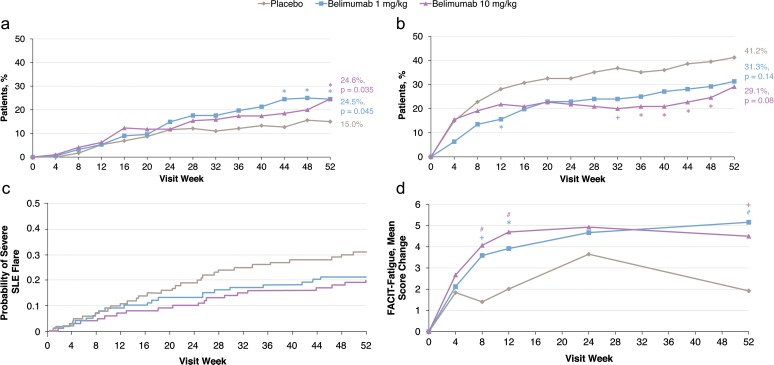
Figure 3.
BLISS trial secondary endpoints in pooled low complement/anti-dsDNA-positive subgroup. (a) Proportions of patients with reduction in corticosteroid dose to 7.5 mg/day or less in patients receiving more than 7.5 mg/day at baseline (n=556). (b) Proportions of patients with increase in corticosteroid dose to more than 7.5 mg/day in patients receiving 7.5 mg/day or less at baseline (n=320). (c) Time to first severe flare (n=876). Hazard ratio (95% CI; p value) versus placebo: 0.67 (0.48–0.94; 0.02) for belimumab 1 mg/kg and 0.61 (0.44–0.85; 0.004) for belimumab 10 mg/kg. (D) Mean change in Functional Assessment Of Chronic Illness Therapy (FACIT)–Fatigue score (n=858). SLE, systemic lupus erythematosus. *p<0.05; +p<0.01; #p<0.001.