Abstract
Background
Prostate cancer is frequently treated with radiotherapy. While treatment results are in general excellent, some patients relapse and current systemic therapies are not curative, thus, underlining the need for novel targeted therapies. Proteasome inhibitors have been suggested as promising new agents against solid tumors including prostate cancer but initial results from clinical trials are disappointing.
Methods
In this study we tested if prostate cancer cells are heterogeneous with regard to their intrinsic 26S proteasome activity, which could explain the lack of clinical responses to bortezomib. PC-3 and DU145 prostate cancer cells and an imaging system for proteasome activity were used to identify individual cells with low proteasome activity. Clonogenic survival assays, a sphere-forming assay and an in vivo limiting dilution assay were used to characterize radiation sensitivity, self-renewal capacity and tumorigenicity of the different subsets of cells
Results
We identified a small population of cells with intrinsically low 26S proteasome activity. Fractionated radiation enriched for these cells and clonogenic survival assays and sphere-forming assays revealed a radioresistant phenotype and increased self-renewal capacity.
Conclusions
We conclude that low 26S proteasome activity identifies a radioresistant prostate cancer cell population. This population of cells could be responsible for the clinical resistance of advanced prostate cancer to proteasome inhibitors and radiation.
Keywords: proteasome, radioresistance, self-renewal, tumorigenicity
Introduction
Cancer of the prostate continues to be a leading cause for cancer deaths in men (1) and is usually treated with radiotherapy alone or in combination with surgery (2). Treatment results are in general excellent (3). However, some patients relapse locally and/or systemically, indicating that a resistant population of cancer cells may have survived the radiation treatment. When prostate cancers progress and metastasize, the tumors frequently become hormone-refractory and classical chemotherapy regimens do not offer a curative approach. Thus, there is a need for novel targeted therapies in advanced prostate cancer (4).
The ubiquitin-proteasome system is the major non-lysosomal system for degradation of intracellular proteins (5). Its activity is fundamental for many cellular processes, such as cell cycle regulation, gene expression, cell differentiation, and immune response (6). Experimental data suggested, that the proteasome could be a novel target in prostate cancer (7,8). However, in first clinical trials the FDA-approved proteasome inhibitor bortezomib had only little anti-tumor activity against prostate cancer (9–13).
We previously reported that low intrinsic proteasome activity in glioma and breast cancer cells correlated with resistance to proteasome inhibitors and radiation (14,15). We hypothesized that the malignant cells in prostate cancer are heterogeneous and that a radioresistant cell population with intrinsically low 26S proteasome activity can also be found in prostate cancer. To address this hypothesis we assessed proteasome activity in cells from two commonly used prostate cancer lines and characterized their radiation response and tumorigenicity.
Material and Methods
Cell culture, reagents, and antibodies
Human PC-3 and DU145 cell lines were purchased from ATCC (Manassas, VA) and cultured under standard conditions as monolayers in DMEM media supplemented with 5% antibiotics (Invitrogen, Carlsbad, CA) and 10% heat inactivated fetal bovine serum (FBS, Sigma, St. Louis, MO) or as prostate spheres in phenol-red-free DMEM/F12 media, 0.4% BSA (Sigma), B27 (Invitrogen), 5 μg/mL bovine insulin (Sigma), 4 μg/mL Heparin (Sigma), 20 ng/mL fibroblast growth factor 2 (Sigma) and 20 ng/mL epidermal growth factor (Sigma). Prostate spheres were initiated from single cells seeded at a density of 10,000 cells/mL. DMEM media, antibiotics, and trypsin were purchased from Invitrogen. PC-3 and DU145 cell lines were transduced with the ZsGreen-cODC proteasome function reporter system as described previously (16). Briefly, the viral expression vector in which the C-terminal degron of the murine ornithine decarboxylase (cODC) was fused to ZsGreen were constructed as follows: The degron coded by the carboxyl-terminal 37 amino acids of ODC fused to ZsGreen (ZsGreen-cODC) was digested with BglII and NotI from pZsProsensor-1 (BD Biosciences, San Jose, CA) and cloned into the BamHI and EcoRI sites of the retroviral vector pQCXIN (BD Biosciences) using the NotI-EcoRI DNA oligonucleotide adaptor (EZCLONE Systems, New Orleans, LA). pQCXIN/ZsGreen-cODC was transfected into GP2-293 pantropic retroviral packaging cells (BD Biosciences). The retrovirus collected from the supernatant of the packaging cells was used to infect the different cell lines. Stable transfectants were selected with G418 (Invitrogen).
To determine that the cells not accumulating the ZsGreen-cODC protein in untreated cell cultures still contained the expression vector, the cells were incubated with 0.5 μM of the proteasome inhibitor MG-132 (Calbiochem, San Diego, CA) overnight and the accumulation of the ZsGreen-cODC protein due to proteasome inhibition was analyzed by flow cytometry.
In all other experiments, accumulation of ZsGreen-cODC protein was analyzed by fluorescence microscopy (Olympus IX71 inverted fluorescent microscope) or flow cytometry (MACSquant analyzer, Miltenyi Biotec GmbH, Auburn, CA). In flow cytometry experiments cells were defined as “ZsGreen-cODC-positive” if the fluorescence in the FL-2 channel (FITC) exceeded the fluorescence of non-transfected control by at least two orders of magnitude.
Determining radiosensitivity of cells with low and high proteasome activity
Monolayer and prostate sphere cultures were plated at a density of 400,000 cells/well or 10,000 cells/mL, respectively, in 6-well plates. Twenty-four hours after plating, cells were irradiated once a day for 5 days with 0, 1, 2, 3, 4, or 5 Gy using an experimental 200KV X-Ray irradiator (Gulmay Medical Ltd., Camberley, England). For each fraction size (5×1 Gy, 5×2 Gy, 5×3 Gy, 5×4 Gy, 5×5 Gy) the total number of ZsGreen-cODC-negative and -positive cells was determined by flow cytometry 24, 48, and 72h after the last irradiation dose. Control cells were sham-irradiated.
Clonogenic Survival Assay and Sphere-forming Assays
For clonogenic survival assays, cells derived from monolayers were irradiated as single cell suspensions. After irradiation, an appropriate number of cells was plated into 10cm Petri dishes into DMEM media, supplemented with 10% FBS. Three weeks later, cells were fixed with methanol, stained with crystal violet and colonies cells were counted. In order to assess sphere formation, cells derived from spheres were irradiated as single cell suspensions, and plated into ultra-low adhesion 96-well plates at clonogenic densities from 1 – 256 cells/well in 100μl of sphere media. Three weeks later, the number of spheres per well was counted. Data points were fitted using a linear-quadratic model.
Primary and Secondary Sphere Formation Assay
PC-3 and DU145 cells expressing ZsGreen-cODC were grown in sphere media as sphere cultures (primary spheres) and sorted into ZsGreen-cODC-negative and -positive cell populations by FACS into ultra low adhesion 96-well plate at a density of one cell per well in DMEM/F12 supplemented with 0.4% BSA (Sigma), 10 ml/500mL B27 (Invitrogen), 5 μg/mL bovine insulin (Sigma), 4 μg/mL heparin (Sigma), 20 ng/mL fibroblast growth factor 2 (bFGF, Sigma) and 20 ng/mL epidermal growth factor (EGF, Sigma). After three weeks, the number of spheres formed per plate were counted and expressed as a percentage of the initial number of cells plated. Cells were also plated in sphere media into 100 mm suspension dishes at 10,000 cells/ml, and allowed to form spheres for 15 days, these cells were used for secondary sphere forming experiments.
For both primary and secondary sphere formation, three independent experiments were performed.
Tumorigenicity and in vivo imaging
Six to eight-week-old male nude (nu/nu) mice originally from The Jackson Laboratories (Bar Harbor, ME) were re-derived, bred, and maintained in a defined flora environment in the animal facilities of the Department of Radiation Oncology, University of California, Los Angeles (Los Angeles, CA) in accordance with all local and national guidelines for the care of animals.
PC-3-ZsGreen-cODC cells were sorted by FACS into ZsGreen-cODC-negative and -positive cells. 106, 105, 104, 103, 102, 10 ZsGreen-cODC-negative cells or -positive cells per inoculum were injected in Matrigel (BD Bioscience) into the thighs. Mice injected with ZsGreen-cODC-negative and -positive cells were imaged for the presence of ZsGreen-cODC-positive cells with the Maestro In Vivo Imaging System (Cambridge Research & Instrumentation, Woburn, MA) before being sacrificed. Tumor growth was monitored on a daily basis and mice were sacrificed when tumor diameters reached the criteria for euthanasia.
Statistical methods
All data are represented as means ± standard error means (SEMs). In general, a P-value of ≤0.05 in a paired two-sided Student's t-test was used to test for statistically significant differences.
Results
Radiation response of prostate cancer cells with high or low proteasome activity
Two commonly used prostate cancer cell lines, PC-3 and DU145, were stably infected with an expression vector for a fusion protein between the green fluorescent protein, ZsGreen, and the C-terminal degron of murine ornithine decarboxylase (cODC). This sequence targets the fusion protein for ubiquitin-independent degradation by the proteasome (14). Cells with low proteasome activity accumulate the fluorescent fusion protein and can be detected by fluorescent microscopy or flow cytometry.
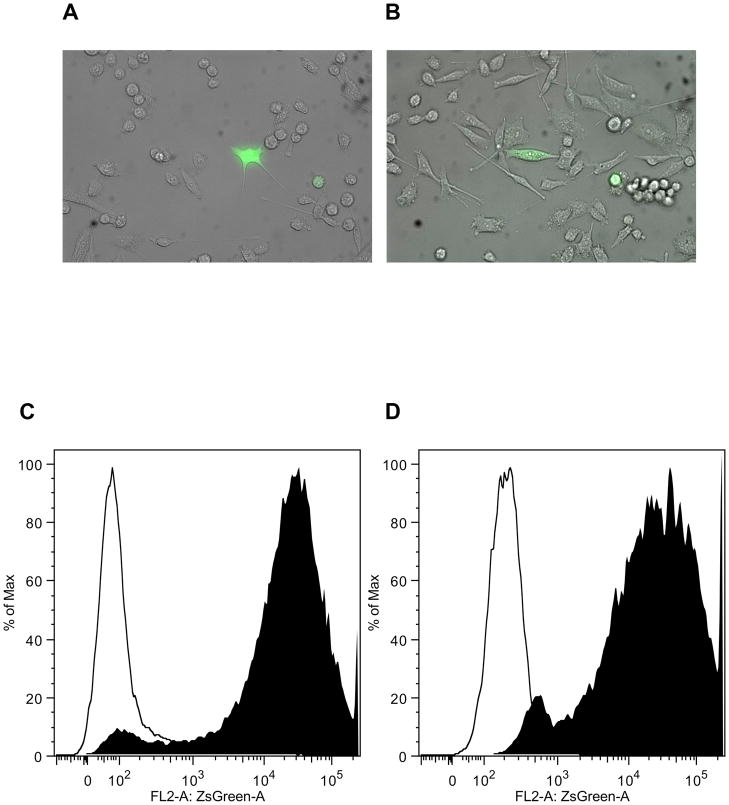
In both cell lines, a small population of cells (PC-3: 2.5% ± 1.3; DU-145: 2.3% ± 0.6) accumulated the reporter protein ZsGreen-cODC, indicating low proteasome function (Figure 1A/B). However, when cells were incubated with the proteasome inhibitor MG-132 (0.5 μM over night), all cells accumulated the fusion protein thus, indicating stable expression of the construct in all cells (Figure 1C/D).
Figure 1.
PC-3 (A) and DU145 (B) prostate cancer lines contain a small population of cells with intrinsically low proteasome activity. Composite images (phase contrast and green fluorescence) of cells with stably trasfected with am expression vector coding for a fusion protein between the green fluorescent protein ZsGreen and the C-terminal degron of murine ornithine decarboxylase (cODC). Accumulation of the fusion protein indicates lack of 26S protesome function. When PC-3-ZsGreen-cODC (C) and DU145-ZsGreen-cODC (D) cells were incubated with the proteasome inhibitor MG-132 (0.5 μM) over night, all cells accumulated the fusion protein.
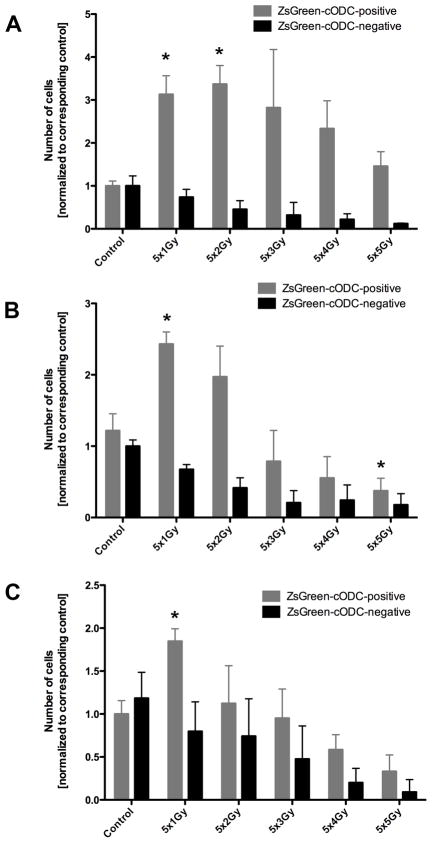
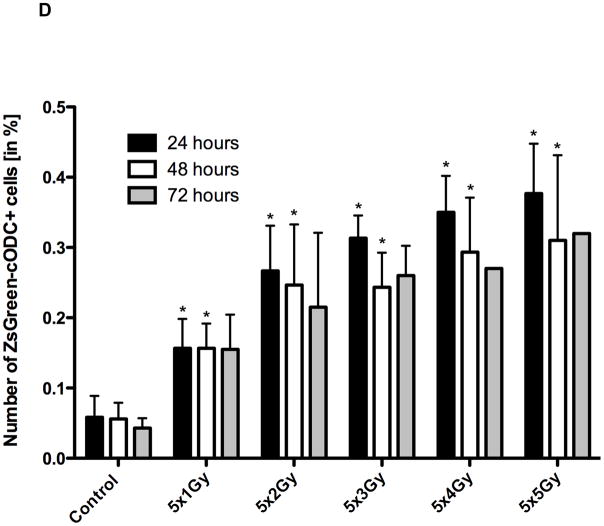
Next we tested if cells accumulating the ZsGreen-cODC reporter (low proteasome activity) could be enriched by irradiation. PC-3 cells were irradiated with 5×1, 5×2, 5×3, 5×4, or 5×5 Gy and the number of ZsGreen-cODC-negative and positive cells was assessed 24, 48, and 72 hours after the last radiation dose (Figure 2A, B, C). Fractionated irradiation increased the absolute and relative number of ZsGreen-cODC-positive cells (low proteasome activity) significantly (5×2Gy: 3fold ± 0.12 n=4, P<0.001, paired two-sided Student’s t-test), while the number of ZsGreen-cODC-negative cells declined (5×2Gy: 0.45fold ± 0.03, n.s., paired two-sided Student’s t-test), indicating differential radiation sensitivity of both cell populations. The increase persisted when cells were analyzed at 48 and 72 hours after the last fraction, supporting preferential killing of cells with high 26S proteasome activity by ionizing radiation (Figure 2E).
Figure 2.
Number of ZsGreen-cODC-negative and ZsGreen-cODC-positive cells, 24, (A) 48 (B), and 72 hours (C) after 5 daily fractions of radiation. Clinically used fractions of 2Gy cause a significant increase in the number of cells with low proteasome activity (ZsGreen-cODC-positive) while the number of cells with high proteasome activity declines (* p<0.05, Student's t-test). (D) percentage of ZsGreen-cODC-positive cells at 24, 48, and 72 hours after tha last fraction of radiation.
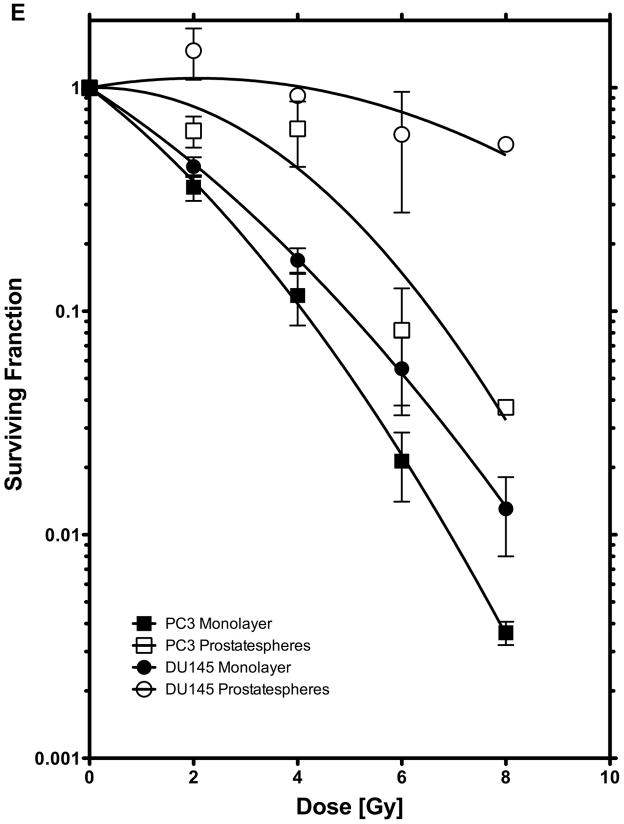
(E) Clonogenic survival and survival of sphere-forming cells after singles doses of radiation. Sphere-forming cells have a radioresistant phenotype.
To compare the radiation sensitivity of clonogenic cells from monolayer cultures with that of sphere-forming cells we performed clonogenic survival assays and sphere forming capacity assays with cells irradiated with 0, 2, 4, 6, or 8 Gy (Figure 2F). The radiation sensitivity of cells cultured as monolayers was comparable between DU-145 and PC-3 cells. However, cells able to initiate prostate spheres exhibited a highly radioresistant phenotype.
Self-renewal capacity and tumorigenicity of prostate cancer cell subpopulations
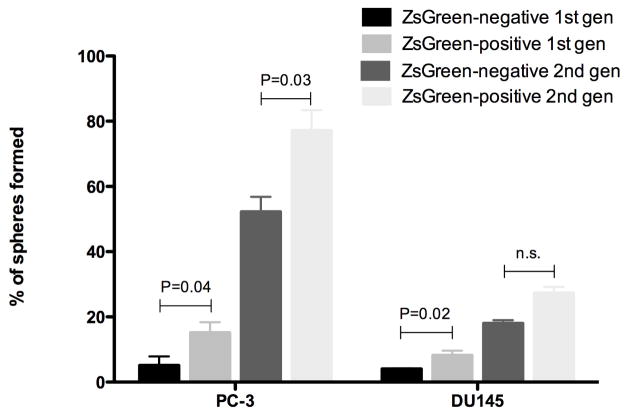
To further investigate differences between prostate cancer cells with high and low proteasome activity we studied their self-renewal capacity and tumorigenicity using an in vitro sphere-forming assay and an in vivo limiting dilution assay. Sphere forming capacity assays were performed by growing PC-3-ZsGreen-cODC cells in monolayer cultures and sorting them in ZsGreen-cODC-negative or -positive cells by fluorescence activated cell sorting (FACS) at a density of 1 cell/well into 96-well ultra-low adhesion plates. After 3 weeks, the number of prostate spheres formed per plate was counted and expressed as a percentage of the initial number of cells plated. Three independent experiments were undertaken, twelve 96-well plates were used per each experiment. ZsGreen-cODC-positive cells had statistically significant higher sphere forming capacity than ZsGreen-cODC-negative cells. In PC-3, 15% of the ZsGreen-cODC-positive cells and only of the 5% ZsGreen-cODC-negative formed primary spheres (P=0.04). In DU145, 8% of the ZsGreen-cODC-positive population and only 4% of the ZsGreen-cODC-negative (P=0.02) formed primary spheres (Figure 2). The secondary sphere formation assays performed in PC-3 or DU145 showed a higher secondary sphere-forming capacity for cells with low proteasome activity compared to the ZsGreen-cODC-negative population (Figure 3).
Figure 3.
Primary and secondary sphere formation from sorted ZsGreen-cODC-negative and -positive cells. In both PC-3 and DU145, ZsGreen-cODC-positive cells showed increased sphere-formation.
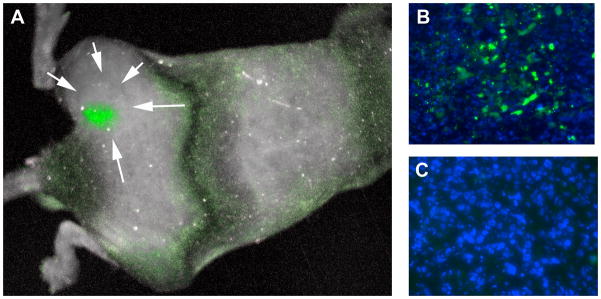
In order to investigate the tumorigenicity of these two subpopulations, PC-3-ZsGreen-cODC cells were sorted by FACS into ZsGreen-cODC-negative and -positive cells and injected subcutaneously into the thighs of 6–8 week-old male Nu/Nu mice. When TD50 values (the number of cells required to form a tumor in 50% of the animals) were calculated, ZsGreen-cODC-positive showed 1 log lower TD50 values than ZsGreen-cODC-negative cells (8.6 × 102 vs. 9.7 × 103). However, this difference was statistically not significant (paired two-sided Student’s t-test). In vivo imaging of the tumors revealed that cells with low proteasome activity were unevenly distributed throughout the tumor and that ZsGreen-cODC-positive cells redistributed into cells with high and cells with low proteasome activity. In contrary, cells with low proteasome activity did not produce progeny with low proteasome activity (Figure 3).
Discussion
In our previous work we reported excellent anti-tumor activity of proteasome inhibitors against a variety of solid tumors including prostate cancer (8). However, when proteasome inhibitors were used against solid cancers in clinical trials, clinical responses were rather disappointing (9–13). The reasons for this failure are unknown. In the present study we tested the hypothesis that prostate cancer cells are heterogeneous with regard to the activity of the 26S proteasome, the target of proteasome inhibitors. We used two established prostate cancer cell lines and an imaging system for proteasome activity to test this hypothesis and to characterize these cells.
We found that a small population of prostate cancer cells accumulated the ZsGreen-cODC reporter protein in the absence of any treatment indicating intrinsically low proteasome function. Similar results were previously reported for glioma, breast, and lung cancer cells (14,15,17). Like breast cancer and glioma cells with low proteasome activity (14,15), those prostate cancer cells were more radioresistant than the bulk tumor cell population and fractionated radiation enriched for these cells. A radioresistant phenotype has bee recently reported for breast cancer cells with low proteasome subunit expression (18). This suggested that if this population of cells also existed in clinical samples it could drive recurrences after radiation treatment. Consistent with this hypothesis, cells with low proteasome activity showed increased self-renewal capacity in vitro. However, tumorigenicity measured by TD50 values of cells with low proteasome activity, did not significantly exceed that of cells with high proteasome activity, indicating that prostate cancer cells with low proteasome activity were not enriched for tumor-initiating cells. However, xenografts could be generated from as few as 100 ZsGreen-cODC-positive and 1,000 ZsGreen-cODC-negative cells respectively, indicating a high frequency of tumor-initiating cells in established prostate cancer cell lines.
Conclusions
We conclude that established prostate cancer cell lines are heterogeneous with regard to their intrinsic proteasome activity and that they contain a radioresistant subpopulation of cells that can be identified by low proteasome activity. The existence of this subpopulation of cells in clinical samples needs to be established in future studies. If it exists it may explain the lack of clinical responses when bortezomib is used against advanced hormone-refractory prostate cancer.
Figure 4.
(A) In vivo imaging of a PC-3 xenograft formed by sorted ZsGreen-cODC-positive cells (white arrows). ZsGreen-cODC-positive cells are not uniformly distributed throughout the tumor but cluster in groups. Sections of tumors formed by ZsGreen-cODC-positive (B) and -negative (C) cells. ZsGreen-cODC-positive cells redistribute into ZsGreen-cODC positive and negative cells while progeny of ZsGreen-negative cells all have high proteasome activity (ZsGreen-cODC-negative).
Acknowledgments
FP was supported by grants of the Department of Defense (W81XWH-07-1- 0065) and the National Cancer Institute (CA137110-01).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2010 doi: 10.1002/cncr.25477. [DOI] [PubMed] [Google Scholar]
- 3.Alicikus ZA, Yamada Y, Zhang Z, Pei X, Hunt M, Kollmeier M, Cox B, Zelefsky MJ. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117(7):1429–1437. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 4.Stavridi F, Karapanagiotou EM, Syrigos KN. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat Rev. 2010;36(2):122–130. doi: 10.1016/j.ctrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Pajonk F, McBride WH. The Proteasome in Cancer Biology and Treatment. Radiat Res. 2001;156(5):447–459. doi: 10.1667/0033-7587(2001)156[0447:tpicba]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Coux O. The 26S proteasome. Prog Mol Subcell Biol. 2002;29:85–107. doi: 10.1007/978-3-642-56373-7_6. [DOI] [PubMed] [Google Scholar]
- 7.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62(18):5230–5235. [PubMed] [Google Scholar]
- 8.Pajonk F, van Ophoven A, Weissenberger C, McBride WH. The proteasome inhibitor MG-132 sensitizes PC-3 prostate cancer cells to ionizing radiation by a DNA-PK-independent mechanism. BMC Cancer. 2005;5(1):76. doi: 10.1186/1471-2407-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris MJ, Kelly WK, Slovin S, Ryan C, Eicher C, Heller G, Scher HI. A phase II trial of bortezomib and prednisone for castration resistant metastatic prostate cancer. J Urol. 2007;178(6):2378–2383. doi: 10.1016/j.juro.2007.08.015. discussion 2383–2374. [DOI] [PubMed] [Google Scholar]
- 10.LoConte NK, Thomas JP, Alberti D, Heideman J, Binger K, Marnocha R, Utecht K, Geiger P, Eickhoff J, Wilding G, Kolesar J. A phase I pharmacodynamic trial of bortezomib in combination with doxorubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2008;63(1):109–115. doi: 10.1007/s00280-008-0719-5. [DOI] [PubMed] [Google Scholar]
- 11.Hainsworth JD, Meluch AA, Spigel DR, Barton J, Jr, Simons L, Meng C, Gould B, Greco FA. Weekly docetaxel and bortezomib as first-line treatment for patients with hormone-refractory prostate cancer: a Minnie Pearl Cancer Research Network phase II trial. Clin Genitourin Cancer. 2007;5(4):278–283. doi: 10.3816/CGC.2007.n.004. [DOI] [PubMed] [Google Scholar]
- 12.Dreicer R, Petrylak D, Agus D, Webb I, Roth B. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(4):1208–1215. doi: 10.1158/1078-0432.CCR-06-2046. [DOI] [PubMed] [Google Scholar]
- 13.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, Kim J, Adams J, Elliott P, Esseltine D, Petrusich A, Dieringer P, Perez C, Logothetis CJ. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22(11):2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 14.Vlashi E, Kim K, Dealla Donna L, Lagadec C, McDonald T, Eghbali M, Sayre J, Stefani E, McBride W, Pajonk F. In-vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101(5):350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagadec C, Vlashi E, Della Donna L, Meng Y, Dekmezian C, Kim K, Pajonk F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010;12(1):R13. doi: 10.1186/bcr2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101(5):350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan J, Zhang Q, Wang Y, You M. 26S proteasome activity is down-regulated in lung cancer stem-like cells propagated in vitro. PLoS One. 2010;5(10):e13298. doi: 10.1371/journal.pone.0013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith L, Qutob O, Watson MB, Beavis AW, Potts D, Welham KJ, Garimella V, Lind MJ, Drew PJ, Cawkwell L. Proteomic identification of putative biomarkers of radiotherapy resistance: a possible role for the 26S proteasome? Neoplasia. 2009;11(11):1194–1207. doi: 10.1593/neo.09902. [DOI] [PMC free article] [PubMed] [Google Scholar]