Abstract
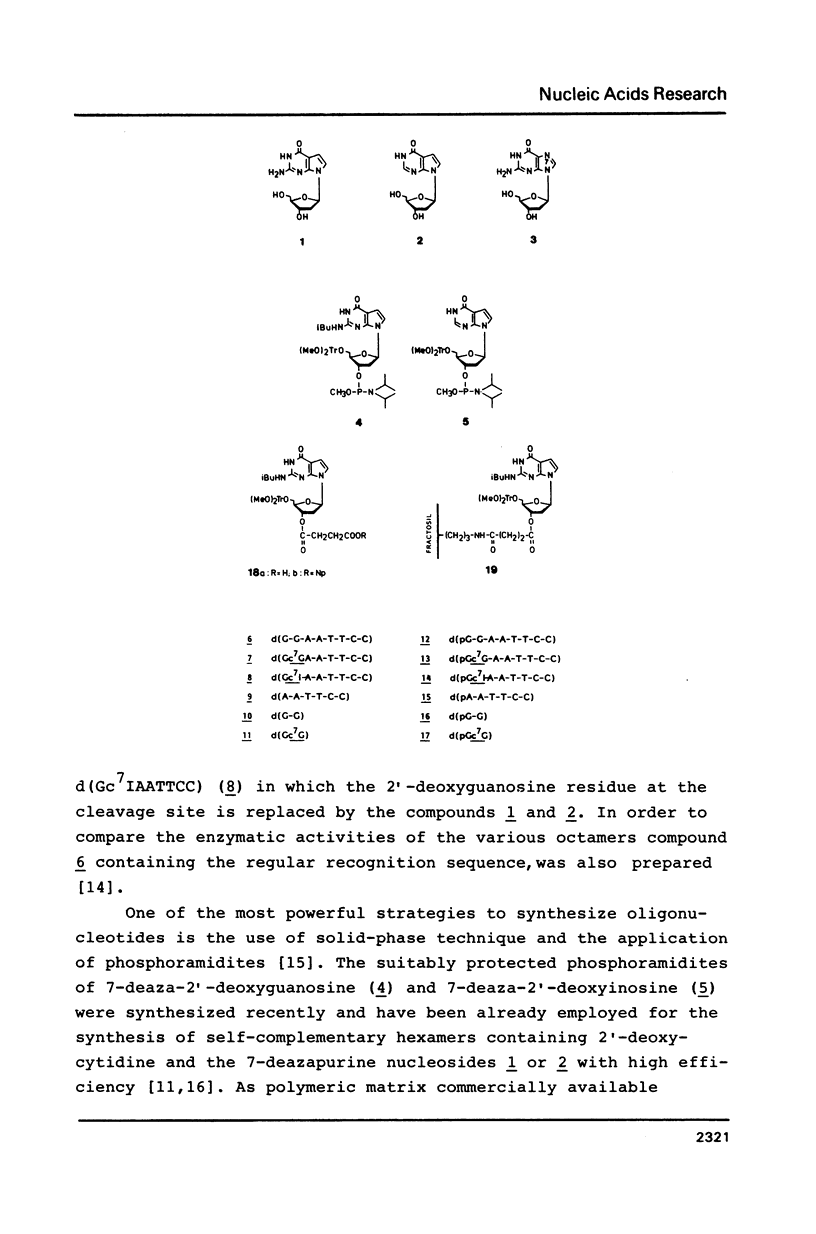
Octadeoxynucleotides with the sequence d[(p)GG*AATTCC] have been prepared by solid-phase synthesis employing regular and base-modified phosphoramidites. These oligomers which contain an isosterically altered recognition sequence of the endodeoxyribonuclease Eco RI form duplexes under appropriate salt conditions. Since G* can represent 7-deaza-2'-deoxyguanosine the oligomers were used as probes to study their cleavage by the endodeoxyribonuclease Eco RI. The enzymatic hydrolysis of the modified octamer was strongly decreased compared to the regular DNA-fragment. This shows that guanine N-7 located at the cleavage site is important for the recognition process by the enzyme. The residual enzymatic activity is discussed on the basis of reduced specificity towards the recognition fragment. The fact that this cleavage occurs already under regular conditions indicates that the process described here bases on an intrinsic property of the oligomer and is different from the star activity.
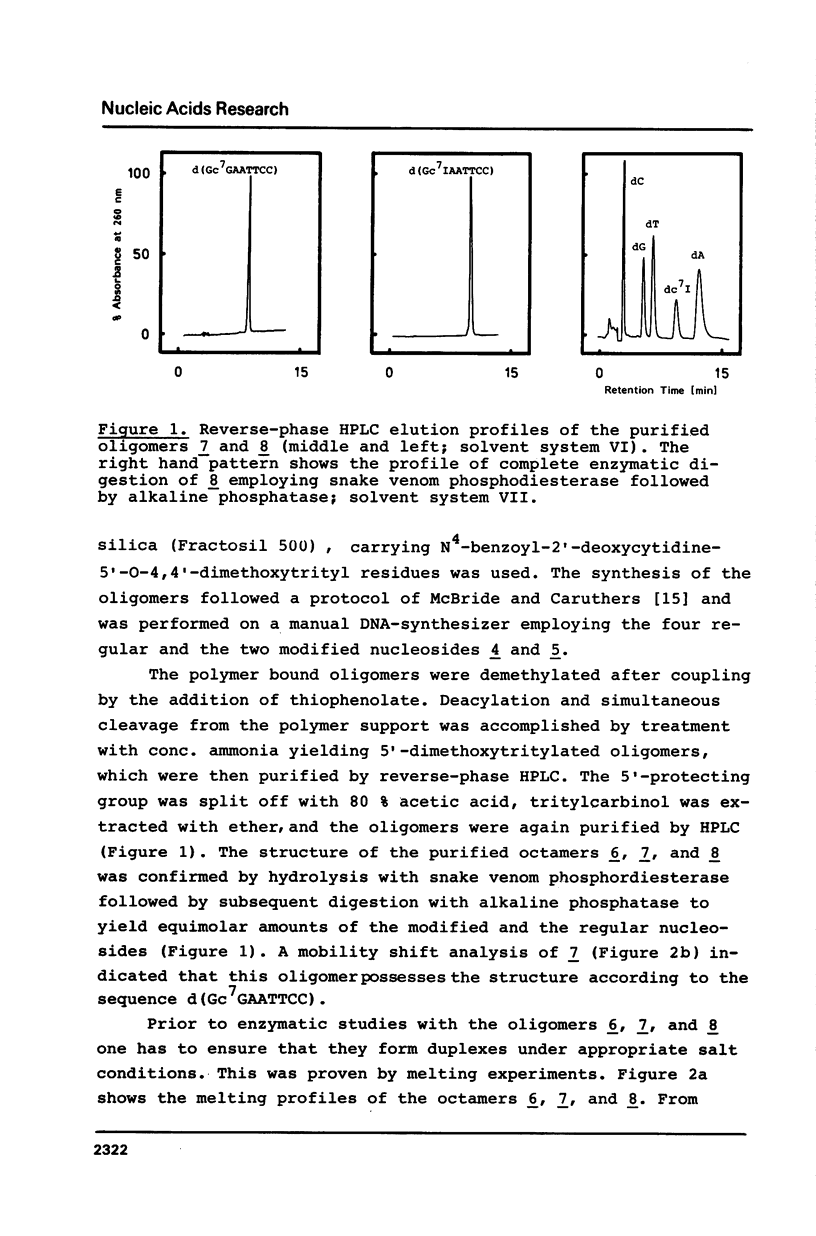
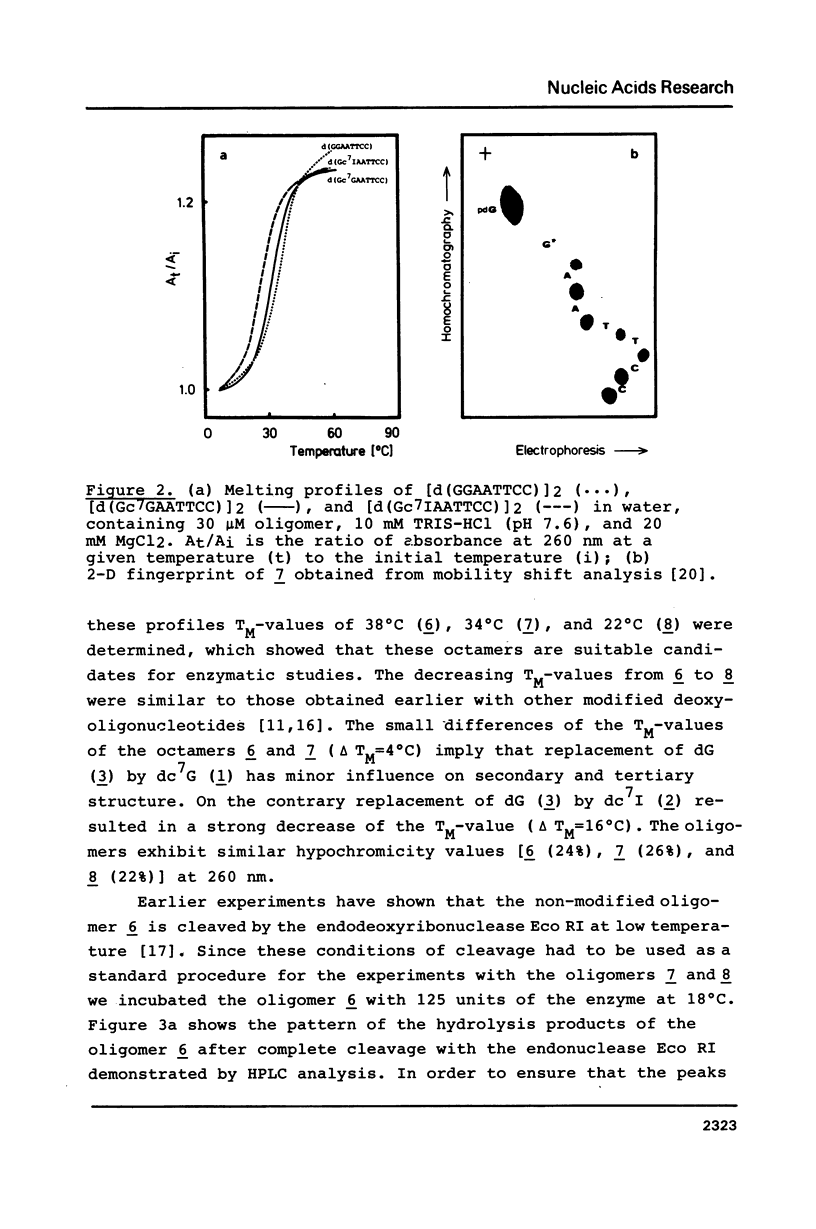
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodnar J. W., Zempsky W., Warder D., Bergson C., Ward D. C. Effect of nucleotide analogs on the cleavage of DNA by the restriction enzymes AluI, DdeI, HinfI, RsaI, and TaqI. J Biol Chem. 1983 Dec 25;258(24):15206–15213. [PubMed] [Google Scholar]
- Connolly B. A., Eckstein F. Assignment of resonances in the 31P NMR spectrum of d(GGAATTCC) by regiospecific labeling with oxygen-17. Biochemistry. 1984 Nov 6;23(23):5523–5527. doi: 10.1021/bi00318a022. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Dwyer-Hallquist P., Kézdy F. J., Agarwal K. L. Interaction of the HpaI endonuclease with synthetic oligonucleotides. Biochemistry. 1982 Sep 14;21(19):4693–4700. doi: 10.1021/bi00262a027. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Grable J., Melia M., Samudzi C., Jen-Jacobson L., Wang B. C., Greene P., Boyer H. W., Rosenberg J. M. Kinked DNA in crystalline complex with EcoRI endonuclease. Nature. 1984 May 24;309(5966):327–331. doi: 10.1038/309327a0. [DOI] [PubMed] [Google Scholar]
- Goppelt M., Pingoud A., Maass G., Mayer H., Köster H., Frank R. The interaction of the EcoRI restriction endonuclease with its substrate. A physico-chemical study employing natural and synthetic oligonucleotides and polynucleotides. Eur J Biochem. 1980 Feb;104(1):101–107. doi: 10.1111/j.1432-1033.1980.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Hsu M., Berg P. Altering the specificity of restriction endonuclease: effect of replacing Mg2+ with Mn2+. Biochemistry. 1978 Jan 10;17(1):131–138. doi: 10.1021/bi00594a019. [DOI] [PubMed] [Google Scholar]
- Lu A. L., Jack W. E., Modrich P. DNA determinants important in sequence recognition by Eco RI endonuclease. J Biol Chem. 1981 Dec 25;256(24):13200–13206. [PubMed] [Google Scholar]
- Marky L. A., Blumenfeld K. S., Kozlowski S., Breslauer K. J. Salt-dependent conformational transitions in the self-complementary deoxydodecanucleotide d(CGCAATTCGCG): evidence for hairpin formation. Biopolymers. 1983 Apr;22(4):1247–1257. doi: 10.1002/bip.360220416. [DOI] [PubMed] [Google Scholar]
- Modrich P., Rubin R. A. Role of the 2-amino group of deoxyguanosine in sequence recognition by EcoRI restriction and modification enzymes. J Biol Chem. 1977 Oct 25;252(20):7273–7278. [PubMed] [Google Scholar]
- Modrich P., Zabel D. EcoRI endonuclease. Physical and catalytic properties of the homogenous enzyme. J Biol Chem. 1976 Oct 10;251(19):5866–5874. [PubMed] [Google Scholar]
- Ohtsuka E., Morisawa H., Ikehara M. Studies on deoxynucleic acids and related compounds. IV. Syntheses of an octanucleotide containing a recognition site for restriction enzyme Eco RI and of an arabinosyladenine analog. Chem Pharm Bull (Tokyo) 1982 Mar;30(3):874–880. doi: 10.1248/cpb.30.874. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Helix-coil transition of the self-complementary dG-dG-dA-dA-dT-dT-dC-dC duplex. Eur J Biochem. 1979 May 15;96(2):267–276. doi: 10.1111/j.1432-1033.1979.tb13037.x. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Driller H. Solid-phase synthesis of the self-complementary hexamer d(c7GpCpc7GpCpc7GpC) via the O-3'-phosphoramidite of 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1985 Feb 11;13(3):911–926. doi: 10.1093/nar/13.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Wu R. Sequence analysis of short DNA fragments. Methods Enzymol. 1980;65(1):620–638. [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- Woodbury C. P., Jr, Hagenbüchle O., von Hippel P. H. DNA site recognition and reduced specificity of the Eco RI endonuclease. J Biol Chem. 1980 Dec 10;255(23):11534–11548. [PubMed] [Google Scholar]


