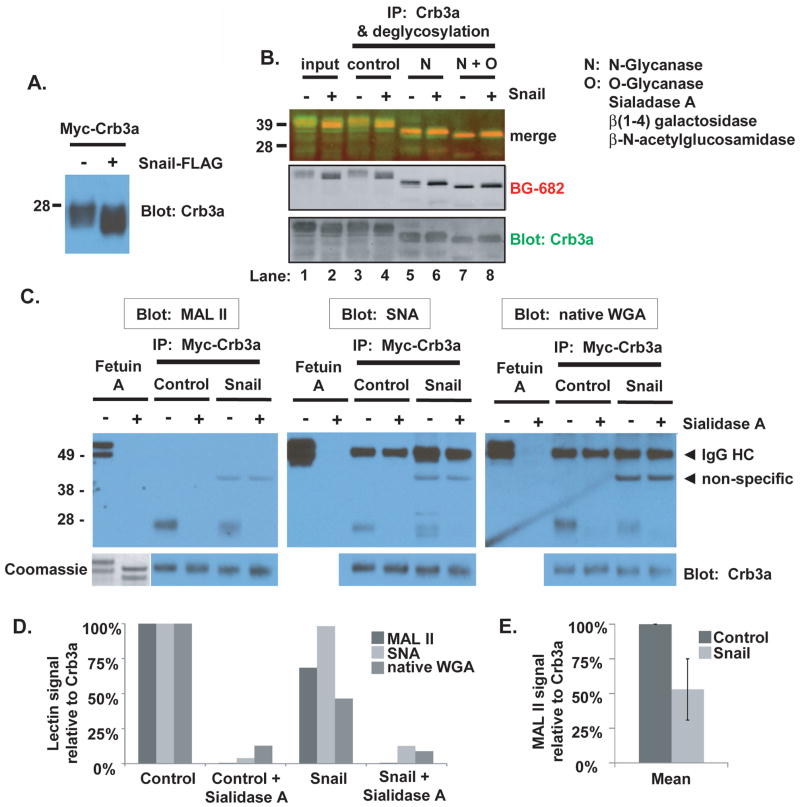
Figure 8. Snail induces differential post-translational modifications of Crumbs3a.
A) Mobility of myc-Crb3a is altered in the presence of Snail as revealed by Crb3a immunoblot of lysates from cells co-expressing myc-Crb3a and either Snail-FLAG (+) or vector control (−). B) Glycosylation pattern analysis of SNAP-Crb3a in the presence or absence of Snail. Following cell surface in-culture labeling with BG-682, SNAP-Crb3a was immunoprecipitated from cell lysates with anti-Crb3a antibody and subjected to N- and O-deglycosylation. SNAP-Crb3a was detected with affinity purified anti-Crb3a antibody. C) Sialylation pattern analysis of SNAP-Crb3a in the presence of absence or Snail by lectin blot. Myc-Crb3a was immunopurified using myc antibody-conjugated beads, denatured and incubated with Sialidase A or control. Following standard PAGE and protein transfer, membranes were blotted with indicated lectins. All membranes were stripped and reprobed with anti-Crb3a antibody. Fetuin A was included as a glycoprotein control; protein presence following desialylation was confirmed in gel by Coomassie staining (lower left corner). D) Graph showing the relative band intensities of the lectin blots in (C), analyzed by densitometry as described in Methods, normalized to Crb3a signal, and reported relative to untreated control cells. E) Graph of average MAL II lectin blot signal intensity as in (C) and (D) obtained from two separate experiments, reported with standard deviations (bars).