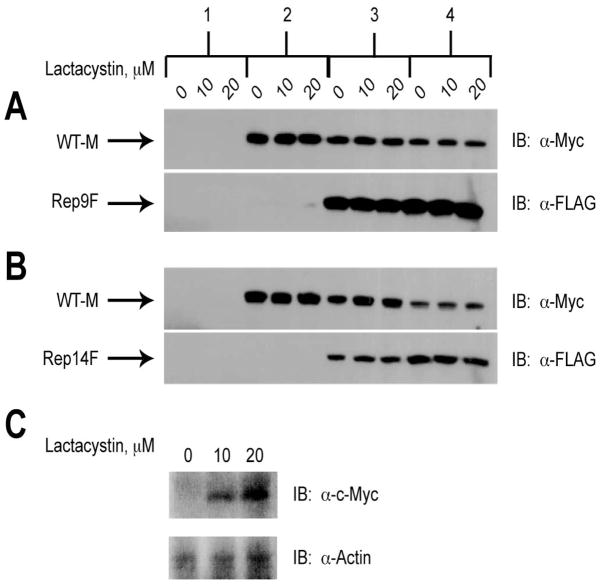
Figure 7. Lack of Effect of Lactacystin on Degradation of Wild-type M6P/IGF2R in Response to Co-expression with Truncated Constructs.
HEK 293 cells were transfected with the cDNA constructs as indicated along the top of the panel: 1) 25 μg pCMV5 vector control; 2) 5 μg WT-M cDNA; 3) 5 μg WT-M + 5 μg mini-receptor cDNAs; 4) 5 μg WT-M + 15 μg mini-receptor cDNAs. Vector control DNA was also added in samples 2 and 3 to normalized DNA transfected to 25 μg per dish. Twenty-four hours after transfection, the cells were treated with the indicated concentrations of lactacystin for 16 h to inhibit proteasomal activity. Detergent extracts were prepared and 100 μg of total protein from each lysate condition was resolved by SDS-PAGE on 6% gels, immunoblotted with α-FLAG or α-Myc antibodies, and developed by autoradiography. Arrows represent the various forms of the M6P/IGF2R expressed in HEK 293 cells. Blots shown are representative of three transfections as follows: co-transfection of A) WT-M and Rep9F or B) WT-M and Rep14F. C) Positive control showing the effect of lactacystin on expression of c-Myc (α-Myc antibody at 1:500). Within each transfection group, there was no significant effect of lactacystin on the intensities of the WT-M bands: A and B) P >0.05 in all comparisons.