Abstract
Background & Rationale
Induction of reactive oxygen species (ROS) is a central mechanism in alcohol hepatotoxicity. Krüppel-like factor 6 (KLF6), a transcription factor and a tumor-suppressor gene, is an early responsive gene to injury; however, the impact of ROS and alcohol on KLF6 induction is unknown.
Aim
To investigate the contribution of two sources of ROS, cytochrome P450 2E1 (CYP2E1) and NAD(P)H quinone oxidoreductase (NQO1) and alcohol to the modulation of KLF6Full expression, splicing to KLF6_V1 and KLF6_V2 and the effect on TNFα, a downstream target.
Methods & Results
Endogenous ROS production in CYP2E1-expressing HepG2 cells induced mRNA and protein expression of KLF6Full and its splice variants compared to control cells. Incubation with pro-oxidants such as arachidonic acid (AA), β-naphtoflavone and H2O2, further enhanced KLF6Full and itssplice variants. The AA effects on KLF6Full and its splice forms were blocked by vitamin E -which prevents lipid peroxidation- and by diallylsulfide -a CYP2E1 inhibitor-. Menadione and paraquat –two pro-oxidants metabolized via NQO1- induced KLF6Full mRNA in a thiol-dependent manner. Antioxidants and a NQO1 inhibitor suppressed the menadione-dependent increase in KLF6Full and its splice variants mRNA. Furthermore, primary hepatocytes and livers from chronic alcohol-fed rats, with elevated lipid peroxidation, H2O2 and CYP2E1 but with low GSH, showed a ~2-fold increase in KLF6Full mRNA compared to controls. Inhibition of p38 phosphorylation further up-regulated the CYP2E1 and the AA effects on KLF6Full mRNA, whereas inhibition JNK and ERK1/2 phosphorylation decreased both. KLF6_V1 but not KLF6Full ablation markedly increased TNFα levels in macrophages; thus, TNFα emerges as a downstream target of KLF6_V1.
Conclusion
The novel effect of ROS on modulating KLF6Full expression and its splice variants could play a relevant role in liver injury and in TNFα regulation.
Keywords: cytochrome P450 2E1, NAD(P)H quinone oxido-reductase, reactive oxygen species, TNFα
INTRODUCTION
Oxidative stress and the generation of ROS are major pathways mediating ethanol hepatoxicity and liver injury. The major interest on CYP2E1 is due to the ability of this uncoupled enzyme to metabolize ethanol, to generate reactive products from ethanol oxidation, to activate many toxicologically relevant agents such as carbon tetrachloride, acetaminophen and alcohols to more toxic and reactive products, to produce ROS and to be induced by alcohol itself (Koop, 1992; Song, 1996; Tanaka et al., 2000).
Induction of CYP2E1 by ethanol and the subsequent state of oxidative stress is a central mechanism leading to altered gene transcription through activation of transcription factors (Bondy, 1992; Cederbaum, 1989; Cederbaum, 2001; Ishii et al., 1997; Lieber, 2000; Nordmann, 1994; Nordmann et al., 1992; Tsukamoto, 2001). Moreover, antioxidant and/or ROS-responsive elements in the promoter of genes are sensitive to oxidative stress and ROS modify mRNA stability (Sen et al., 2005; Zhao et al., 2004). In contrast to these well-known pathways of transcriptional and post-transcriptional control, nothing is known on whether CYP2E1 and ethanol-induced ROS could modulate mRNA splicing, which is emerging as a critical regulator of tissue repair and differentiation.
Zinc finger proteins have appeared as a major class of eukaryotic transcription factors. The Krüppel-like family of zinc finger transcription factors regulate cell growth, proliferation, apoptosis and angiogenesis (Bieker, 2001; Black et al., 1999; Dang et al., 2000; Kaczynski et al., 2003; Oates et al., 2001; Philipsen and Suske, 1999). All KLF family members possess a highly-conserved C-terminal 81 amino acid Zinc finger DNA-binding domain that can interact with ‘GC-box’ or ‘CACC-box’ DNA motifs in responsive promoters, with each KLF having a distinct N-terminal activation domain (Philipsen and Suske, 1999). While the DNA binding domains of KLFs are identical, their divergent activation domains account for their broad range of biologic activities. KLFs can homo-or heterodimerize with either other KLFs (Merika and Orkin, 1995) or with heterologous transcription factors (Sur et al., 2002; Zhang et al., 2002).
KLF6 is a ubiquitously expressed Krüppel-like transcription factor (Ho and Piquette-Miller, 2007; Sirach et al., 2007a) and a tumor-suppressor gene (Narla et al., 2005; Narla et al., 2001; Narla et al., 2007) whose role in vivo has not been fully clarified. Known putative transcriptional targets are placental glycoprotein (Blanchon et al., 2001), COL1A1 (Ratziu et al., 1998), TGFβ (Kim et al., 1998), types I and II TGFβ receptors (Kim et al., 1998) and the urokinase type plasminogen activator (Kojima et al., 2000). In humans, KLF6 can undergo splicing to shorter isoforms (KLF6_V1, KLF6_V2 and KLF6_V3) (Narla et al., 2005) (Suppl. Fig. 1), which lack all or part of the DNA binding domain. Inducers and repressors of KLF6 as well as the repertoire of downstream targets, particularly targets that may play a key role in liver injury, are not fully characterized to date (Bieker, 2001). Little is known on how KLF6Full and its splice variants may sense ROS and ethanol, whether a shift in their ratios may occur and whether this could affect their down-stream targets, many of which remain unknown. The possible involvement of ROS and ethanol in the expression of KLF6Full and its splice isoforms may help to understand their potential contribution to tissue injury and repair in alcoholic liver disease and other diseased states, where oxidative stress occurs.
MATERIAL AND METHODS
Cell lines and Primary Hepatocytes
Most of our studies were performed with CYP2E1-expressing cells (E47 cells) generated in the laboratory from Arthur I. Cederbaum (Mount Sinai School of Medicine, NY) (Chen and Cederbaum, 1998; Chen et al., 1997), which are HepG2 cells constitutively expressing human CYP2E1. The control cells (C34 cells) are HepG2 cells transfected with pCI-neo and do not express CYP2E1. The colon cancer HCT116 cells and the murine RAW 246.7 macrophages were obtained from the ATCC (Manassas, VA). Using endotoxin-free collagenase, primary hepatocytes were isolated from rats fed for 8 months, which in humans would be about 20–30 years, with the Lieber-DeCarli diets (Lieber and DeCarli, 1982). The percentage of ethanol-derived calories was 10% for 1 wk, 20% for 1 wk and 35% for 7.5 months. The control group received isocaloric amounts of dextrose (Lieber and DeCarli, 1982). Protocols were approved by the Institutional Animal Care and Use Committee at our Institution. In all cells, CYP2E1 content was measured by Western blot and the catalytic activity by using the fluorescent substrate 7-Methoxy-4-trifluoromethylcoumarin (7-MFC) or by the method of Reinke (Reinke and Moyer, 1985).
Liver Pathology
Blood was collected from the abdominal aorta prior to liver perfusion. Plasma was assayed for the activity of ALT and AST, ethanol concentration and non-esterified fatty acids (NEFA) using kits from Thermo Electron Corporation (Waltham, MA), Sigma (St. Louis, MO) and Wako Chemicals (Richmond, VA), respectively. Liver sections were obtained from the left liver lobe. Samples were fixed overnight in 10% buffered formalin and embedded in paraffin. Sections (5 μm) were stained with H&E and evaluated by a liver pathologist who was blinded from the experimental information.
General Methodology
Cell viability was monitored using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Intra- and extracellular lipid peroxidation end-products were determined from the production of thiobarbituric acid reactive substances (TBARS) with a standard curve of malonaldehyde bisdimethylacetal (Nieto and Rojkind, 2007). Extracellular H2O2 was assessed by the ferrous oxidation-xylenol orange assay (Nourooz-Zadeh et al., 1994). GSH levels were measured in the protein free extract by the recycling method of Tietze (Tietze, 1969). Northern blot analysis for CYP2E1, KLF6Full and GAPDH mRNA was carried out using cDNA probes developed in our laboratory (Nieto et al., 1999). Western blot analysis for CYP2E1 was performed using an antibody kindly donated by Dr. Jerome Lasker (Puracyp Inc., Carlsbad, CA) (Shimizu et al., 1990). The KLF6 antibody, which recognizes all isoforms, was from Santa Cruz Biotechnology (Santa Cruz, CA). The quantification of the intensity of the signal in Western and Northern blots was expressed under the blots as arbitrary units of densitometry.
Flow-Cytometry Analysis
Intracellular H2O2 and O2.− were assessed using the fluorescent probes 2′, 7′-dichlorofluorescein diacetate (DCFDA) and dihydroethidium (DHE) (Brenner et al., 2003), respectively (Molecular Probes, Eugene, OR). TNFα was measured in cells by flow cytometry analysis using anti-rat TNFα PE-conjugated antibody (BD Pharmingen, San Jose, CA). The intensity of fluorescence was quantified using a FACS Calibur™ Flow Cytometer and analyzed using CellQuest Pro™ software (BD Biosciences, San Jose, CA). Secreted TNFα was measured using an ELISA kit (Invitrogen, Carlsbad, CA).
Quantitative Real-Time PCR
Total RNA was extracted using the PureLink Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA), treated with recombinant DNAse (Roche Diagnostic, IN) and reverse transcribed using the FastStart PCR Master Mix (Roche Diagnostic, Indianapolis, IN). Triplicate qRT-PCR reactions were performed with the LightCycler using the primers listed in Table 1. Data were analyzed using the LightCyclerR 480 software normalizing the results by GAPDH.
Table 1.
Primers
| mRNA | Forward primer | Reverse primer | Amplicon size |
|---|---|---|---|
| KLF6Fu ll | 5′-CGGACGCACACAGGAGAAAA-3′ Exon 2-Exon 3 (nt 763–782) | 5′-CGGTGTGCTTTCGGAAGTG-3′ Exon 3 | 103 bp |
| KLF6_V 1 | 5′-CCTCGCCAGGGAAGGAGAA-3′ Exon 2-Exon 3 (nt 509–780) | 5′-CGGTGTGCTTTCGGAAGTG-3′ Exon 3 | 104 bp |
| KLF6_V 2 | 5′-GTCGGGGAAGCCAGAGAA-3′ Exon 2-Exon 3 (nt 607–780) | 5′-CGGTGTGCTTTCGGAAGTG-3′ Exon 3 | 103 bp |
| GAPDH | 5′-CAATGACCCCTTCATTGACC-3′ | 5′-GATCTCGCTCCTGGAAGATG-3′ | 113 bp |
Transfection Experiments and Reporter Assays
RAW 246.7 cells were plated at a density of 5×104/well in 12-well plates 1 day prior to transfection. Co-transfection studies were performed using Lipofectamine reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The human -982-TNFα promoter construct was provided by Dr. Goldfeld (Harvard Medical School, Boston, MA). A total of 1 μg of -982-TNFα promoter construct and pGL3 basic and 50 ng of pRenilla-Luc were transfected. Twenty-four hours later scrambled siRNA, siRNA KLF6Full or siRNA KLF6_V1 (20 pmol) were transfected per well. In some experiments, cells were treated with AA for 3 h before harvesting them. Cells were collected 48 h after the transfection with the TNFα reporter construct and both firefly and renilla luciferase activities were measured.
Statistical Analysis
Data were analyzed by a Student’s t test and by a two-factor ANOVA when applicable. Values are expressed as means ± S.E.M. (N>3).
RESULTS
Overexpression of CYP2E1 -a Source of ROS- Induces KLF6Full and its Splice Forms
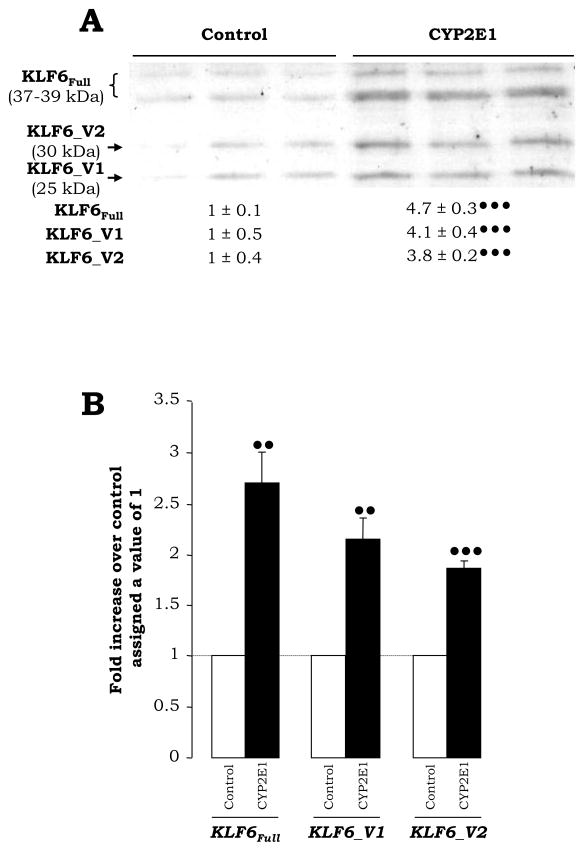
To evaluate the contribution of ROS to the expression of KLF6Full and its spliced forms, an immortalized HepG2 cell line over-expressing CYP2E1 (Fig. 1A–1C), as a source of endogenous production of ROS (Fig. 1D–1F, white bars) was used and found increased expression of KLF6Full (37–39 kDa) and of its splice variants KLF6_V1 (25 kDa) and KLF6_V2 (30 kDa) when compared with control HepG2 cells (Fig. 2A). Similar results were observed at the mRNA and protein level (Fig. 2B, and not shown). This is likely due to elevated CYP2E1-mediated ROS increase as it does not occur in the control HepG2 cells.
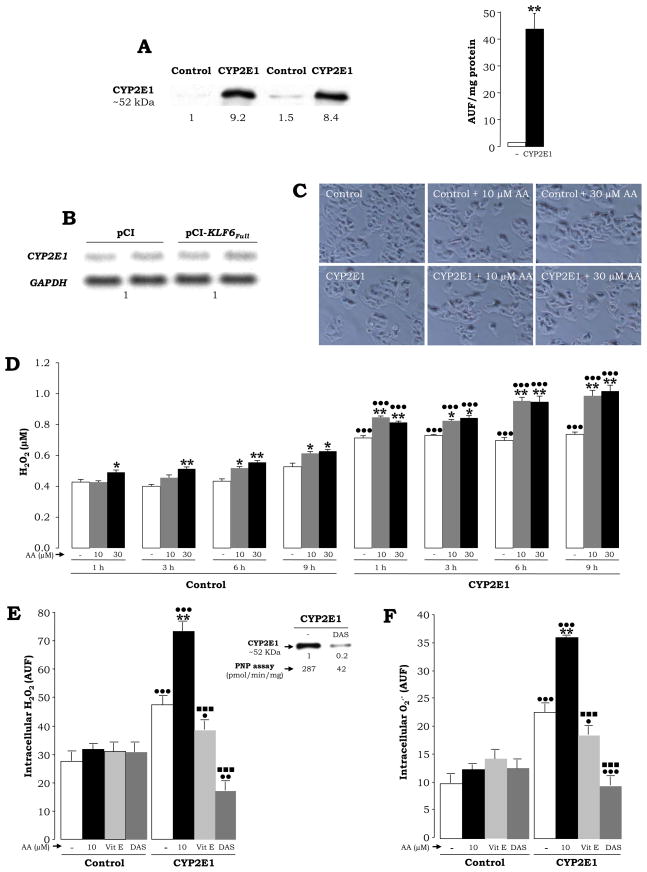
Fig. 1. CYP2E1-Expressing Cells Generate ROS in the Absence or Presence of AA.
Control and CYP2E1-expressing cells were analyzed for CYP2E1 protein by Western blot. The quantification of the signal was referred to the first control, which was assigned a value of 1. CYP2E1 activity was monitored by fluorimetry using 7-MFC as substrate. Results refer to mean ± SEM (N=3, **P<0.01) (A). CYP2E1-expressing cells were transfected with either pCi-Neo or pCi-Neo-KLF6Full vectors and CYP2E1 and GAPDH expression were analyzed by Northern blot (B). Control and CYP2E1-expressing cells were treated with 0–30 μM AA for 6 h (100% viability) and light micrographs were taken (C). Time-course study (1–9 h) of extracellular H2O2 in the presence of 0–30 μM AA (D). Intracellular H2O2 and O2.− at 6 h after 30 μM AA-treatment in the presence of either 25 μM vitamin E or 5 mM diallylsulfide (DAS) using the fluorescent probes DCFDA (E) and DHE (F). Results in (D) to (F) are means ± SEM (N=6; *P<0.05 and **P<0.01 for AA-treated vs. untreated, •P<0.05, •• P<0.01 and •••P<0.001 for CYP2E1-expressing cells vs. control, ■ ■ ■P<0.001 for co-treated vs. AA-treated).
Fig. 2. Expression of KLF6Full and its Splice Variants in CYP2E1-Expressing Cells.
The expression of KLF6Full, KLF6_V1 and KLF6_V2 protein was analyzed by Western blot. The quantification of the signal was referred to the average signal in the controls, which was assigned a value of 1 (A). qRT-PCR for KLF6Full, KLF6_V1 and KLF6_V2 in control and CYP2E1-expressing cells (B). In both panels, results are expressed as fold increase over the control which was assigned a value of 1 (dashed line in (B)) and refer to means ± SEM (N=3, ••P<0.01 and •••P<0.001 for CYP2E1-expressing cells vs. control).
Increased Expression of KLF6Full does not Alter CYP2E1 Levels
Since KLF6 is a transcription factor, to elucidate if there could be a feed-back loop between KLF6Full and CYP2E1, CYP2E1-expressing cells were transfected with the pCI-neo vector containing the cDNA encoding for KLF6Full or with the empty vector and 48 h later CYP2E1 expression was analyzed by Northern blot. Similar CYP2E1 mRNA levels were found in all samples (Fig. 1B). GCLC and GCLM, two proteins that regulate de novo GSH synthesis, which could prevent ROS-induced injury, also remained similar (not shown).
Arachidonic Acid Induces ROS Mostly in CYP2E1-Expressing Cells
To study the role of CYP2E1-derived ROS on KLF6Full expression and splicing, we first confirmed the ability of the CYP2E1-expressing cells to generate a state of oxidant stress in the presence of substrates relevant for the development of alcohol-induced liver injury (Nanji, 2004). Both control and CYP2E1-expressing cells were incubated with 0–30 μM AA -a representative polyunsaturated fatty acid (PUFA)- in a time-course experiment up to 9 h (Fig. 1D–1F, grey and back bars). Short time-points were chosen because KLF6Full is an early-response gene (Kojima et al., 2000). Cell viability was assessed by the MTT assay and by flow cytometry analysis (not shown) or by monitoring for changes in cell morphology; however, no signs of cytotoxicity were observed at the selected time-points (Fig. 1C). CYP2E1-expressing cells showed higher extra- and intracellular H2O2 levels (Fig. 1D–1E, white bars) as well as intracellular O2 − (Fig. 1F, white bars) than controls. ROS generation was further elevated by treatment with AA (Fig. 1D–1F, gray and black bars). CYP2E1 expression and activity can be modulated by addition of specific inhibitors and the effect mediated by ROS can be prevented by antioxidants. Addition of vitamin E -which prevents lipid peroxidation reactions- (Fig. 1E–1F, light grey bars) and of diallylsulfide -a selective CYP2E1 inhibitor- (Fig. 1E–1F, dark grey bars), prevented the increase in intracellular H2O2 and O2 −, respectively. Levels and activity of CYP2E1 under diallylsulfide treatment are shown in the inset.
Pro-oxidants Increase KLF6Full Mainly in CYP2E1-Expressing Cells
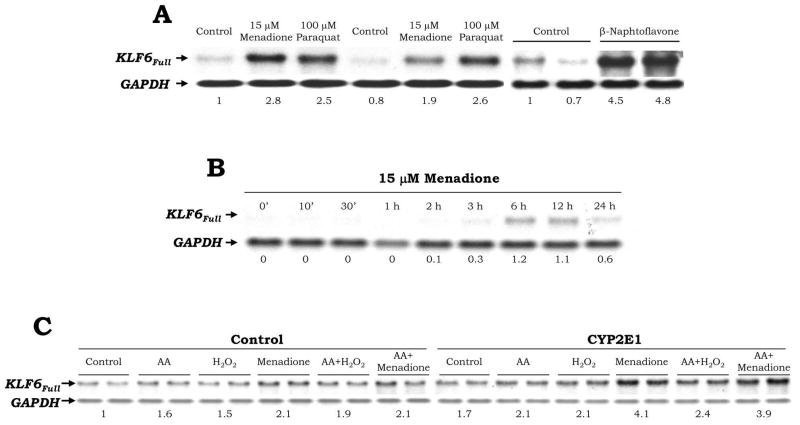
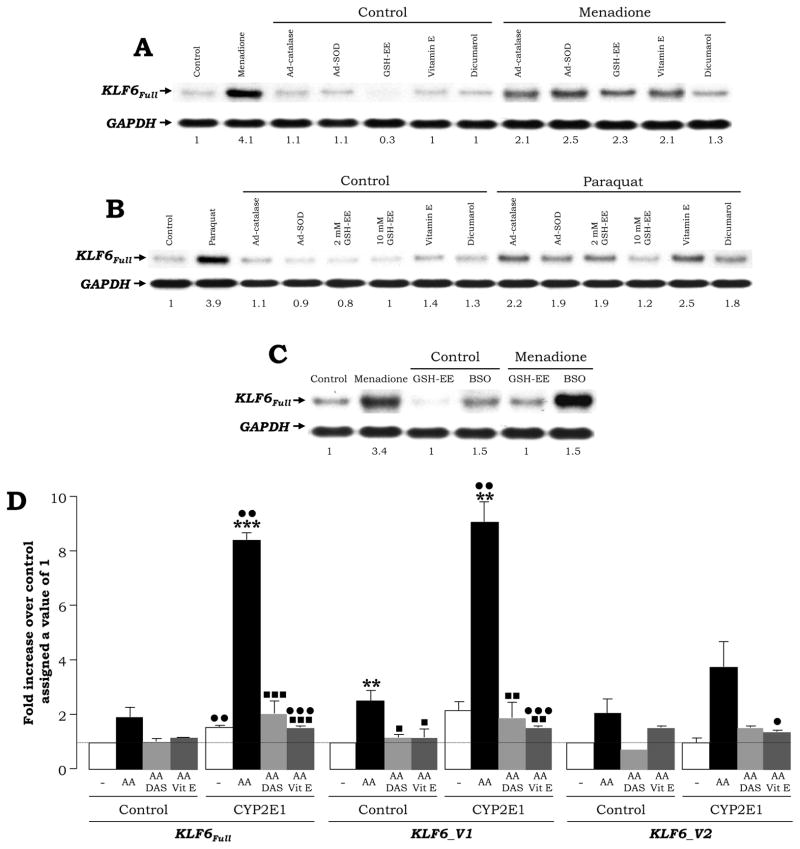
Addition of two pro-oxidants (15 μM menadione or 100 μM paraquat), which undergo two electron reduction through NAD(P)H quinone oxidoreductase, or of a CYP2E1 inducer (100 μM β-naphtoflavone), elevated KLF6Full expression ~1.9-to-4.8-fold in the CYP2E1-expressing cells as shown by Northern blot analysis (Fig. 3A). A time-course experiment was carried out with menadione to define the time-point of maximal induction (6–12 h, Fig. 3B). Treatment of HCT116 cells -a colon cancer cell line- with menadione caused a similar effect on KLF6Full (Not shown). A second set of experiments was carried out in the presence of other pro-oxidants such as 30 μM AA, 20 μM H2O2, 15 μM menadione and the combination of AA plus H2O2 and of AA plus menadione, at doses and times that did not affect cell viability (not shown). There was an induction of KLF6Full expression under all these treatments, which was more apparent for the CYP2E1-expressing cells than for the control cells (Fig. 3C). Addition of iron -a catalyst of lipid peroxidation-derived reactions- to AA-treated cells further increased KLF6Full expression (not shown).
Fig. 3. Pro-oxidants Increase KLF6Full.
CYP2E1-expressing cells were treated in duplicate with 15 μM menadione, 100 μM paraquat or 100 μM β-naphtoflavone for 6 h (A) or in a time-course study up to 24 h with 15 μM menadione (B) and KLF6Full expression was evaluated by Northern blot analysis. A value of 1 was given to the untreated cells in (A) and to the first time-point to show any signal in (B). Control and CYP2E1-expressing cells were treated with 30 μM AA, 20 μM H2O2, 15 μM menadione or the combination of AA plus H2O2 and AA plus menadione for 6 h and KLF6Full expression was assessed by Northern blot analysis. The quantification of the signal indicated under the blots is the average of N=2. The signal for the untreated controls was assigned a value of 1 (C).
Pro-oxidants Also Increase KLF6_V1 and KLF6_V2 mRNA Mainly in CYP2E1-Expressing Cells
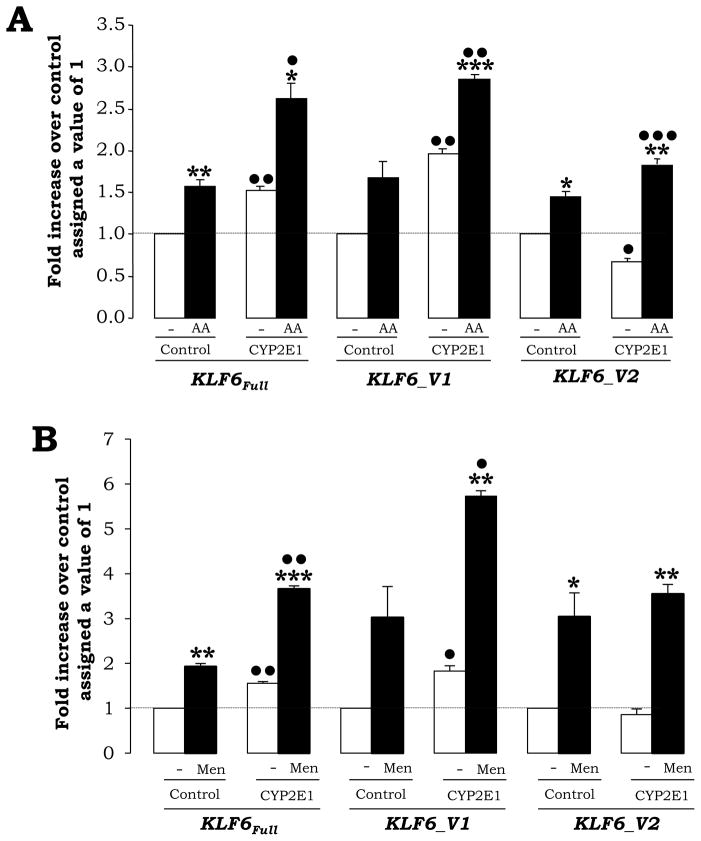
To establish a link between ROS, KLF6Full expression and the possible modulation of the KLF6 splice variants by ROS, control and CYP2E1-expressing cells were incubated with either 30 μM AA or with 15 μM menadione for 6 h and levels of KLF6Full and its splice forms KLF6_V1 and KLF6_V2 were analyzed by qRT-PCR (Fig. 4A–4B). In the absence of treatments, KLF6Full, KLF6_V1 and KLF6_V2 appeared equally induced in the CYP2E1-expressing cells (Fig. 4A–4B, white bars). Addition of AA or menadione further increased mRNA levels of all KLF6 isoforms mainly in the CYP2E1-expressing cells (Fig. 4A–4B, black bars).
Fig. 4. Pro-oxidants Increase KLF6Full and its Splice Forms More in CYP2E1-Expressing Cells than in Controls.
Control and CYP2E1-expressing cells were treated with 30 μM AA (A) or with 15 μM menadione (B) for 6 h and the expression of KLF6Full and its splice forms KLF6_V1 and KLF6_V2 was assessed by qRT-PCR. Results are expressed as fold increase over the untreated controls, which were assigned a value of 1 (dashed line) and are means ± SEM (N=3; *P<0.05, **P<0.01 and ***P<0.001 for treated vs. control and •P<0.05, ••P<0.01 and •••P<0.001 for CYP2E1-expressing cells vs. control).
Antioxidants and Inhibitors of NQO1 or CYP2E1 Prevent the Increase in KLF6Full and its Splice Forms by Menadione, Paraquat and AA
Control and CYP2E1 expressing cells were incubated with 0–15 μM menadione or with 0–100 μM paraquat in the absence or presence of antioxidants (an adenovirus over-expressing catalase or SOD, 2–10 mM glutathione ethyl ester (GSH-EE) and 25 μM vitamin E), pro-oxidants (L-buthionine sulfoximine -a GSH depleting agent-) or a NQO1 inhibitor (25 μM dicumarol). Addition of antioxidants and of a NQO1 inhibitor prevented the increase in KLF6Full by menadione and by paraquat (Fig. 5A–5B). GSH depletion by BSO further elevated the effect of menadione on KLF6Full expression whereas GSH-EE blunted it (Fig. 5C). In addition, cells were incubated with 0–30 μM AA in the absence or presence of diallylsulfide -a CYP2E1 inhibitor- and of vitamin E -an antioxidant-. AA increased 1.8-and 5.3-fold KLF6Full, 2.3- and 4.2-fold KLF6_V1 and 2- and 3.7-fold KLF6_V2 in control and CYP2E1 expressing cells, respectively (Fig. 5D, black bars vs. white bars). This increase was blocked by diallylsulfide (Fig. 5D, light grey bars vs. black bars) and by vitamin E (Fig. 5D, dark grey bars vs. black bars) or by 0.1 mM sodium diethyldithiocarbamate, another CYP2E1 inhibitor (not shown).
Fig. 5. Antioxidants and NQO1 and CYP2E1 Inhibitors Prevent the Increase in KLF6Full and its Splice Forms.
CYP2E1 expressing cells were incubated with 15 μM menadione (A) and with 100 μM paraquat (B) in the absence or presence of adenoviruses expressing catalase or SOD, 2–10 mM GSH-EE, 25 μM vitamin E and 25 μM dicumarol. CYP2E1 expressing cells were also incubated with 15 μM menadione in the presence of 0–0.2 mM BSO (C) and Northern blot analysis were carried out to evaluate for KLF6Full expression. A value of 1 was given to the untreated cells. Control and CYP2E1-expressing cells were incubated with 0–30 μM AA in the presence of 25 μM vitamin E or of 5 mM diallylsulfide (DAS) for 6 h and the expression of KLF6Full and its splice forms KLF6_V1 and KLF6_V2 was assessed by qRT-PCR (D). Results are expressed as fold increase over the untreated controls which were assigned a value of 1 (dashed line) and are means ± SEM (N=3; **P<0.01 and ***P<0.001 for AA-treated vs. control, •P<0.05, ••P<0.01 and •••P<0.001 for CYP2E1-expressing cells vs. control and ■P<0.05, ■ ■P<0.01 and ■ ■ ■P<0.001 for co-treated vs. AA-treated).
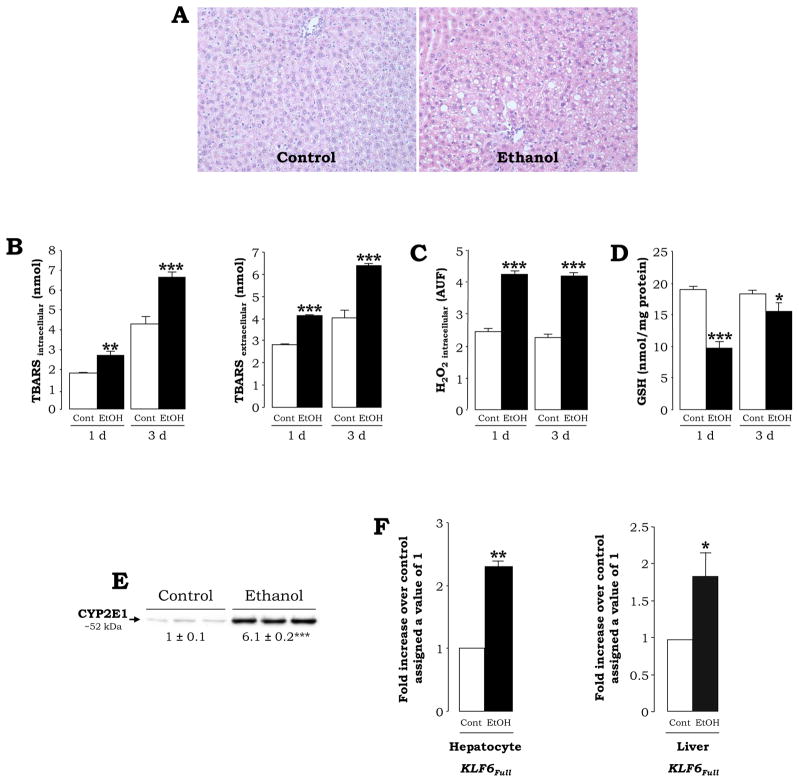
KLF6Full Expression Increases in Primary Hepatocytes from Ethanol-Fed Rats with High CYP2E1
Since alcohol-induced liver injury is mediated in part by ROS, we extended our experiments to primary hepatocytes isolated from rats fed the control or the ethanol Lieber-DeCarli diets, an in vivo model known to induce oxidative stress mostly via CYP2E1 induction. The Lieber-DeCarli diets are a very well established protocol for inducing early alcohol-mediated liver injury (Lieber, 1999) and cause moderate steatosis (Fig. 6A, right panel), a 2-fold increase in AST, ALT, plasma ethanol levels of ~120 mg/dL and a 5-fold increase in non-esterified fatty acids (not shown). Oxidative stress was elevated in hepatocytes from ethanol-fed rats as shown by intra-and extracellular TBARS, intracellular H2O2 and GSH levels (Fig. 6B–6D) and by CYP2E1 expression (Fig. 6E). qRT-PCR analysis indicated induction of KLF6Full in hepatocytes and in total liver from ethanol-fed rats (Fig. 6F). Similar results were obtained in hepatocytes isolated from pyrazole-injected rats, a chemical known to induce CYP2E1 levels (not shown). The spliced variants were not analyzed as KLF6 splicing has not been described in rat.
Fig. 6. Primary Hepatocytes and Livers from Ethanol-Fed Rats show Increased expression of KLF6Full.
H&E staining (A) in rats fed the control Lieber-DeCarli diet showed minimal steatosis (left), while rats fed the ethanol Lieber-DeCarli diet showed periportal and pericentral micro- and macro-vesicular steatosis (right) (original magnification=200x). Levels of intra- and extracellular lipid peroxidation (TBARS) (B), intracellular H2O2 (C) and GSH (D). Western blot analysis for CYP2E1 expression in hepatocytes from control and ethanol-fed rats (E). Induction of KLF6Full expression in hepatocytes and in total liver (F) from ethanol-fed rats was determined by qRT-PCR. Results are expressed as fold-increase over the untreated controls, which were assigned a value of 1. In all panels results are means ± SEM (N=6–10, *P<0.05, **P<0.01 and ***P<0.001 for ethanol vs. control).
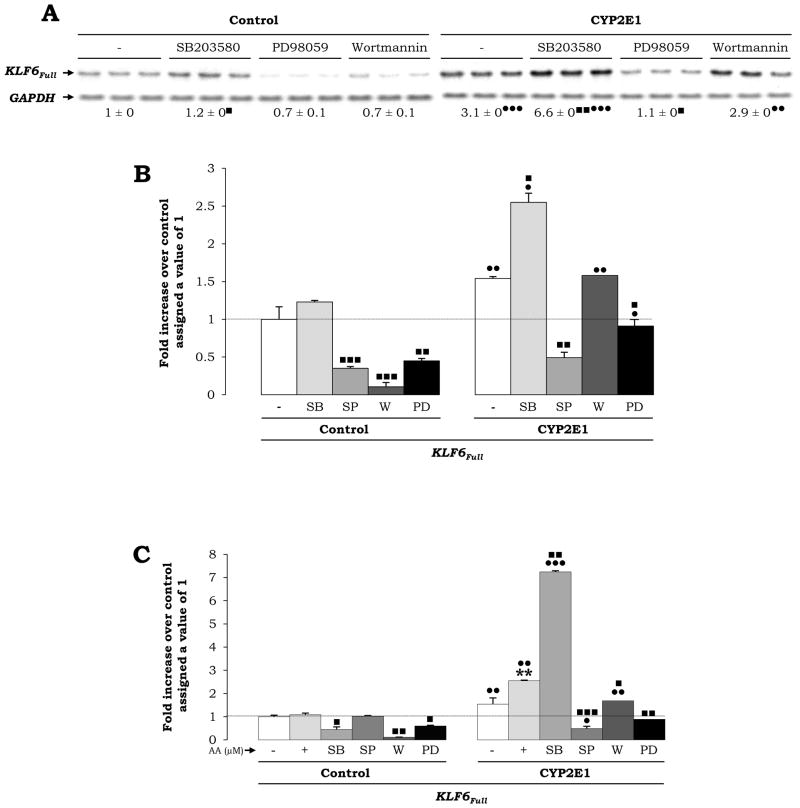
Stress-Activated Kinases Participate in the Up-Regulation of KLF6Full Expression
To determine if there was an association between activation of stress-sensitive kinases and KLF6Full expression, we added specific inhibitors of phosphorylation of kinases. SB203580 -an inhibitor of p38 phosphorylation- increased the expression of KLF6Full whereas PD98059 -an inhibitor of MEK1/2 phosphorylation- or SP600125 -an inhibitor of JNK phosphorylation- significantly decreased KLF6Full in CYP2E1 expressing cells compared to controls, establishing a link between ROS, stress-activated kinases and KLF6Full levels (Fig. 7A–7B). Moreover, addition of SB203580 2 h prior to treatment with 30 μM AA, further elevated KLF6Full by 2.5-fold over the AA-mediated increase, while no additive effects were observed by SP600125, Wortmanin or PD98059 (Fig. 7C).
Fig. 7. KLF6Full Expression is Regulated by Stress-Activated Kinases.
Control and CYP2E1-expressing cells were incubated in the presence of inhibitors of phosphorylation of p38 (SB203580), MEK1/2 (PD95059) and PI3K (Wortmannin) and the expression of KLF6Full was assessed by Northern blot analysis. Untreated controls were assigned a value of 1 (A). qRT-PCR for KLF6Full in control and CYP2E1-expressing cells incubated with the above indicated inhibitors and with the inhibitor of JNK phosphorylation SP60025 added in the absence (B) or in presence of 30 μM AA (C). In all panels, results are expressed as fold increase over the untreated control cells assigned a value of 1 and refer to means ± SEM. (N=3; **P<0.01 for AA-treated vs. control, •P<0.05, ••P<0.01 and •••P<0.001 for CYP2E1-expressing cells vs. control and ■P<0.05, ■ ■P<0.01 and ■ ■ ■P<0.001 for co-treated vs. AA-treated).
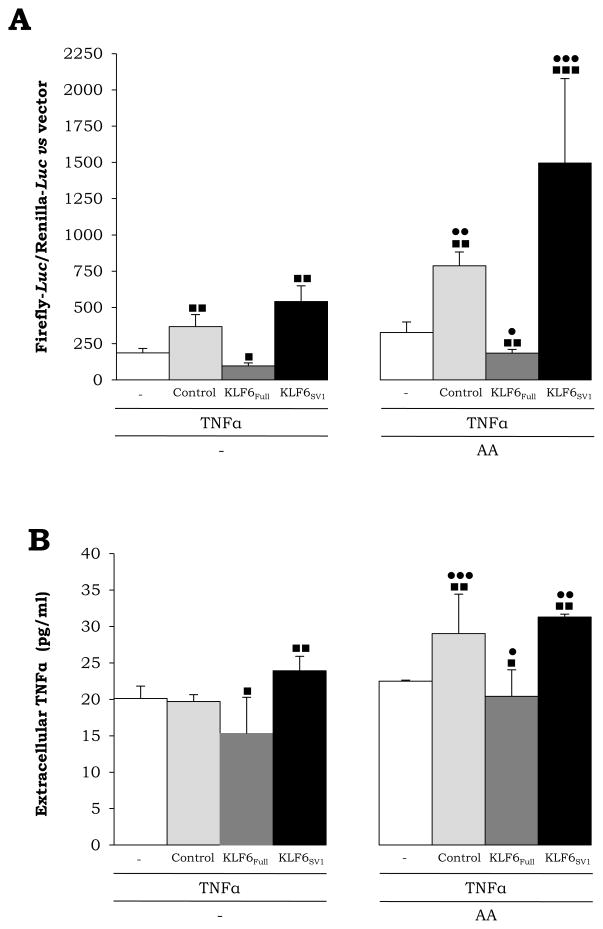
TNFα, a Potential Down-Stream Target of KLF6_V1
To determine whether KLF6 or KLF6_V1 may affect TNFα, which plays a critical role in alcoholic liver disease and in other modes of liver injury, RAW 264.7 cells, a mouse macrophage cell line, were transfected with the −980-TNFα promoter and then cotransfected with control, KLF6Full or KLF6_V1 siRNAs. In some experiments, cells were treated 48 h after transfections with 30 μM AA for 3 h. Transfection efficiency was evaluated and results were normalized to Renilla-Luc. Transfection with KLF6_V1 siRNA caused a 2-fold increase and a 6-fold increase in intracellular TNFα in untreated RAW cells and in cell treated with AA, respectively (Fig. 8A) and a slight increase in secreted TNFα was also observed (Fig. 8B); thus, establishing a connection between KLF6_V1, oxidant stress and TNFα.
Fig. 8. KLF6_V1 Ablation Up-Regulates TNFα in the presence of AA.
RAW 246.7 macrophages were transfected with the -982-TNFα promoter. Then, a co-transfection was carried out with control, KLF6Full or KLF6_V1 siRNAs. In some experiments, cells were treated with 30 μM AA for 3 h. Luc activity after 48 hours of transfection was corrected by transfection efficiency and normalized with Renilla-Luc (A). Secreted TNFα was measured in the respective supernatants by ELISA (B). Results are expressed as mean ± SEM (N=6; ■P<0.05, ■ ■P<0.01 and ■ ■ ■P<0.001 for any siRNA vs. control and •P<0.05, ••P<0.01 and •••P< 0.0 01 f or A A-treated vs. control).
DISCUSSION
Among the mechanisms involved in alcohol toxicity, ROS production is believed to be highly significant and it is currently at the center of considerable research. Generation of ROS, along with a compromised antioxidant defense, can affect gene transcription and the subsequent activation of cellular targets. In addition, a variety of cellular stress signaling pathways can affect the responsiveness to ROS and determine protein function and eventually cell fate (Carreras and Poderoso, 2007). Thus, studies on the effects of ROS on induction of key proteins -such as KLF6- and its potential down-stream targets -such as TNFα- are relevant to better understand the mechanisms of alcohol-induced liver injury and other liver diseases and to develop new strategies for therapy.
To define the impact of ROS on the expression of KLF6Full and its splice variants, we used an immortalized HepG2 cell line over-expressing CYP2E1, as a source of endogenous ROS production, since CYP2E1 is an uncoupled enzyme that metabolizes alcohol and as a result, generates a state of oxidative stress, even in the absence of any added substrate. There was a concurrent increase in the expression of KLF6Full and its splice variants KLF6_V1 and KLF6_V2 in CYP2E1-expressing cells when compared with control cells, suggesting a potential link between ROS and KLF6Full expression and splicing. The possibility that KLF6 -as a transcription factor- could modify ROS production per se was ruled out as transfection with a plasmid containing the cDNA encoding for KLF6Full neither affected CYP2E1 expression nor nitric oxide synthase 2 (NOS2), GCLC, GCLM or catalase.
We next explored the contribution of two pro-oxidants to the induction of KLF6Full mRNA. Menadione and paraquat are redox cycling compounds, which undergo two-electron reduction through NQO1, generating non-alkylating metabolites that react with O2 to produce ROS, mostly O2.−, H2O2, OH− and OH. Addition of menadione or paraquat induced KLF6Full mRNA as did β-naphtoflavone -a cytochrome P450 inducer-. Similar results were observed with menadione in the colon cancer cell line HCT116, suggesting that the effects of these pro-oxidants may not be limited to the liver. Moreover, pro-oxidants whose mechanism of action differ such as AA, H2O2 and menadione either added alone or in combination, triggered an increase in KLF6Full mRNA mostly in the CYP2E1 expressing cells than in the corresponding controls, suggesting that pro-oxidants either per se (e.g. H2O2), via CYP2E1-metabolism (e.g. AA, β-naphtoflavone) or through NQO1 metabolism (e.g. menadione and paraquat) enhance KLF6Full mRNA. Furthermore, both AA and menadione induced not only KLF6Full mRNA but also triggered a parallel up-regulation of KLF6_V1 and KLF6_V2 mRNA, mostly in CYP2E1-expressing cells, likely reflecting a transcriptional effect rather than increased alternative splicing.
Since this in vitro model allows regulating ROS levels, to validate the role of ROS in modulating KLF6 mRNA, next we incubated cells in the presence of antioxidants and selective inhibitors of the specific source of ROS. Transduction with adenoviruses containing the cDNA encoding either for catalase -to decompose H2O2- or for SOD1 -to dismutate O2.−- or treatment with vitamin E -to block lipid peroxidation-derived reactions-partially prevented the increase in KLF6Full mRNA by menadione and by paraquat. Likewise, dicumarol -an inhibitor of NQO1- blunted the effects mediated by menadione and paraquat. Similar results were observed in cells incubated with AA in the presence of vitamin E or diallylsulfide -a CYP2E1 inhibitor- validating the role of CYP2E1 in the transcriptional effects triggered by AA on KLF6Full and its splice variants.
Cellular GSH plays an essential role in preventing damage of oxidative nature to cellular membranes and acts as a hydrogen donor for glutathione peroxidases, which represent a line of defense against lipid peroxidation. Agents that lower GSH, such as L-buthionine sulfoximine, induce cell damage by events related to oxidative stress associated with decrease in protein thiols. Depletion of GSH increased the sensitivity of KLF6Full mRNA to menadione. Conversely, restoration of the GSH pool with GSH-EE blunted the increase in KLF6Full mRNA by menadione, suggesting a direct link between KLF6Full and GSH. It is possible that the menadione effects on KLF6 are preceded by depletion of intracellular GSH stores due to oxidation to GSSG or to a decrease in protein thiols, likely due to oxidation of –SH groups, although arylation may also occur. The redox transitions of KLF6 –SH groups may affect its activity and cellular function.
As a second approach, we then extended our studies to primary hepatocytes isolated from rats fed the control or the ethanol Lieber-DeCarli diets, a model of early alcohol-induced liver injury. Hepatocytes from ethanol-fed rats displayed increased lipid peroxidation, elevated H2O2 and lower GSH, along with induced CYP2E1. KLF6Full mRNA was analyzed in hepatocytes and in total liver and found to be elevated about two-fold in ethanol-fed rats. Since KLF6Full has not been described to undergo alternative splicing in rats, the splice variants were not analyzed. KLF6Full up-regulation in primary hepatocytes from chronic ethanol-fed rats may reflect an adaptive mechanism to protect against CYP2E1-derived oxidant stress. It could be postulated that in alcohol-induced liver injury, where an oxidative burst occurs due to CYP2E1 up-regulation and alcohol metabolism, modulation of KLF6 expression contributes to cell defense. Splicing of KLF6, at least in humans, may be an early event in alcohol-induced liver injury, leading to changes in KLF6 expression and activation of protein kinases that contribute to liver damage and/or repair. In addition to alcoholic liver disease, KLF6 has been associated with non-alcoholic liver disease (Starkel et al., 2003) and hepatocellular carcinoma (Sirach et al., 2007b; Tarocchi et al., 2011; Zhenzhen et al., 2011).
Since KLF6Full expression appeared regulated by ROS and many protein kinases are highly responsive under oxidant stress conditions, we studied the role of stress-activated kinases to the ROS modulation of KLF6Full. Addition of an inhibitor of p38 phosphorylation increased KLF6Full mRNA whereas inhibitors of JNK or of MEK1/2 decreased KLF6Full mRNA. Since AA induced KLF6Full mRNA and SB203580, an inhibitor of p38 phosphorylation has been shown to prevent AA toxicity in the CYP2E1 expressing cells (Wu and Cederbaum, 2003), we next analyzed whether p38 phosphorylation could play a role in modulating KLF6Full mRNA levels. Incubation of cells with SB203580 prior to AA addition further increased KLF6Full mRNA levels, likely contributing to cellular defense; while addition of SP60025 or PD98059, inhibitors of phosphorylation of JNK or MEK1/2 barely lowered KLF6Full mRNA expression.
Because of the essential role of TNFα in alcoholic liver disease, we next investigated whether TNFα could be a target of either KLF6Full or KLF6_V1, the main KLF6 isoforms induced by oxidant stress. Knock down of KLF6Full slightly decreased TNFα promoter transactivation and blunted the response to AA. In contrast, KLF6_V1 ablation increased TNFα promoter transactivation, which was further enhanced by AA. This finding suggests that TNFα may be a direct target of KLF6_V1. These results were also validated in human stellate cells, which also have the ability to generate TNFα upon the onset of liver injury (not shown). In conclusion, these results indicate that several sources of endogenous ROS increase KLF6Full expression and its splice variants; thus, contributing to modulating a critical downstream target such as TNFα.
Supplementary Material
Acknowledgments
Financial support: Raquel Urtasun received a Postdoctoral Fellowship from the Government of Navarre (Spain). Francisco Javier Cubero received a Postdoctoral Fellowship from the Ministry of Education and Science (Spain) (Ref. No. EX2006-0070). US Public Health service grants 5R01 AA017733, 5R01 AA017733-01S1, 5P20 AA017067, 5P20 AA017067-01S1 and 5P20 AA017067-03S1 from the National Institute on Alcohol Abuse and Alcoholism (N.N.).
The authors are very grateful to Arthur I. Cederbaum for his insight during this project. We would like to thank Scott L. Friedman and his laboratory members for their advice in some of the experiments and for facilitating some reagents.
Abbreviations
- AA
Arachidonic acid
- BSO
L-buthionine sulfoximine
- CYP2E1
cytochrome P450 2E1
- DAS
diallylsulfide
- GSH
glutathione
- KLF6
Krüppel-like factor 6
- KLF6_V1
KLF6 variant 1
- KLF6_V2
KLF6 variant 2
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NEFA
non-esterified fatty acids
- NOS2
nitric oxide synthase 2
- NQO1
NAD(P)H quinone oxidoreductase
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid-reactive substances
References
- Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276(37):34355–8. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- Black BE, Levesque L, Holaska JM, Wood TC, Paschal BM. Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export. Mol Cell Biol. 1999;19(12):8616–24. doi: 10.1128/mcb.19.12.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchon L, Bocco JL, Gallot D, Gachon AM, Lemery D, Dechelotte P, Dastugue B, Sapin V. Co-localization of KLF6 and KLF4 with pregnancy-specific glycoproteins during human placenta development. Mech Dev. 2001;105(1–2):185–9. doi: 10.1016/s0925-4773(01)00391-4. [DOI] [PubMed] [Google Scholar]
- Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63(3):231–41. doi: 10.1016/0378-4274(92)90086-y. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100(24):13761–6. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras MC, Poderoso JJ. Mitochondrial nitric oxide in the signaling of cell integrated responses. Am J Physiol Cell Physiol. 2007;292(5):C1569–80. doi: 10.1152/ajpcell.00248.2006. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. Role of lipid peroxidation and oxidative stress in alcohol toxicity. Free Radic Biol Med. 1989;7(5):537–9. doi: 10.1016/0891-5849(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. Introduction-serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med. 2001;31(12):1524–6. doi: 10.1016/s0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- Chen Q, Cederbaum AI. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol Pharmacol. 1998;53(4):638–48. doi: 10.1124/mol.53.4.638. [DOI] [PubMed] [Google Scholar]
- Chen Q, Galleano M, Cederbaum AI. Cytotoxicity and apoptosis produced by arachidonic acid in Hep G2 cells overexpressing human cytochrome P4502E1. J Biol Chem. 1997;272(23):14532–41. doi: 10.1074/jbc.272.23.14532. [DOI] [PubMed] [Google Scholar]
- Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32(11–12):1103–21. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EA, Piquette-Miller M. KLF6 and HSF4 transcriptionally regulate multidrug resistance transporters during inflammation. Biochem Biophys Res Commun. 2007;353(3):679–85. doi: 10.1016/j.bbrc.2006.12.090. [DOI] [PubMed] [Google Scholar]
- Ishii H, Kurose I, Kato S. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J Gastroenterol Hepatol. 1997;12(9–10):S272–82. doi: 10.1111/j.1440-1746.1997.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4(2):206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, Kim SJ, Friedman SL. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273(50):33750–8. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- Kojima S, Hayashi S, Shimokado K, Suzuki Y, Shimada J, Crippa MP, Friedman SL. Transcriptional activation of urokinase by the Kruppel-like factor Zf9/COPEB activates latent TGF-beta1 in vascular endothelial cells. Blood. 2000;95(4):1309–16. [PubMed] [Google Scholar]
- Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. Faseb J. 1992;6(2):724–30. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998)--a review. Alcohol Clin Exp Res. 1999;23(6):991–1007. [PubMed] [Google Scholar]
- Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol. 2000;32(1 Suppl):113–28. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6(4):523–31. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Merika M, Orkin SH. Functional synergy and physical interactions of the erythroid transcription factor GATA-1 with the Kruppel family proteins Sp1 and EKLF. Mol Cell Biol. 1995;15(5):2437–47. doi: 10.1128/mcb.15.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34(1):21–5. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Narla G, Difeo A, Reeves HL, Schaid DJ, Hirshfeld J, Hod E, Katz A, Isaacs WB, Hebbring S, Komiya A, McDonnell SK, Wiley KE, Jacobsen SJ, Isaacs SD, Walsh PC, Zheng SL, Chang BL, Friedrichsen DM, Stanford JL, Ostrander EA, Chinnaiyan AM, Rubin MA, Xu J, Thibodeau SN, Friedman SL, Martignetti JA. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Res. 2005;65(4):1213–22. doi: 10.1158/0008-5472.CAN-04-4249. [DOI] [PubMed] [Google Scholar]
- Narla G, Heath KE, Reeves HL, Li D, Giono LE, Kimmelman AC, Glucksman MJ, Narla J, Eng FJ, Chan AM, Ferrari AC, Martignetti JA, Friedman SL. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science. 2001;294(5551):2563–6. doi: 10.1126/science.1066326. [DOI] [PubMed] [Google Scholar]
- Narla G, Kremer-Tal S, Matsumoto N, Zhao X, Yao S, Kelley K, Tarocchi M, Friedman SL. In vivo regulation of p21 by the Kruppel-like factor 6 tumor-suppressor gene in mouse liver and human hepatocellular carcinoma. Oncogene. 2007 doi: 10.1038/sj.onc.1210223. [DOI] [PubMed] [Google Scholar]
- Nieto N, Friedman SL, Greenwel P, Cederbaum AI. CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology. 1999;30(4):987–96. doi: 10.1002/hep.510300433. [DOI] [PubMed] [Google Scholar]
- Nieto N, Rojkind M. Repeated whiskey binges promote liver injury in rats fed a choline-deficient diet. J Hepatol. 2007;46(2):330–9. doi: 10.1016/j.jhep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994;29(5):513–22. [PubMed] [Google Scholar]
- Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12(3):219–40. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994;220(2):403–9. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- Oates AC, Pratt SJ, Vail B, Yan Y, Ho RK, Johnson SL, Postlethwait JH, Zon LI. The zebrafish klf gene family. Blood. 2001;98(6):1792–801. doi: 10.1182/blood.v98.6.1792. [DOI] [PubMed] [Google Scholar]
- Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 1999;27(15):2991–3000. doi: 10.1093/nar/27.15.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman SL. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci U S A. 1998;95(16):9500–5. doi: 10.1073/pnas.95.16.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13(5):548–52. [PubMed] [Google Scholar]
- Sen P, Chakraborty PK, Raha S. p38 mitogen-activated protein kinase (p38MAPK) upregulates catalase levels in response to low dose H2O2 treatment through enhancement of mRNA stability. FEBS Lett. 2005;579(20):4402–6. doi: 10.1016/j.febslet.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Lasker JM, Tsutsumi M, Lieber CS. Immunohistochemical localization of ethanol-inducible P450IIE1 in the rat alimentary tract. Gastroenterology. 1990;99(4):1044–53. doi: 10.1016/0016-5085(90)90625-b. [DOI] [PubMed] [Google Scholar]
- Sirach E, Bureau C, Peron JM, Pradayrol L, Vinel JP, Buscail L, Cordelier P. KLF6 transcription factor protects hepatocellular carcinoma-derived cells from apoptosis. Cell Death Differ. 2007a doi: 10.1038/sj.cdd.4402114. [DOI] [PubMed] [Google Scholar]
- Sirach E, Bureau C, Peron JM, Pradayrol L, Vinel JP, Buscail L, Cordelier P. KLF6 transcription factor protects hepatocellular carcinoma-derived cells from apoptosis. Cell Death Differ. 2007b;14(6):1202–10. doi: 10.1038/sj.cdd.4402114. [DOI] [PubMed] [Google Scholar]
- Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20(8 Suppl):138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- Starkel P, Sempoux C, Leclercq I, Herin M, Deby C, Desager JP, Horsmans Y. Oxidative stress, KLF6 and transforming growth factor-beta up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol. 2003;39(4):538–46. doi: 10.1016/s0168-8278(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Sur I, Unden AB, Toftgard R. Human Kruppel-like factor5/KLF5: synergy with NF-kappaB/Rel factors and expression in human skin and hair follicles. Eur J Cell Biol. 2002;81(6):323–34. doi: 10.1078/0171-9335-00257. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther. 2000;25(3):165–75. doi: 10.1046/j.1365-2710.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- Tarocchi M, Hannivoort R, Hoshida Y, Lee UE, Vetter D, Narla G, Villanueva A, Oren M, Llovet JM, Friedman SL. Carcinogen-induced hepatic tumors in KLF6+/- mice recapitulate aggressive human hepatocellular carcinoma associated with p53 pathway deregulation. Hepatology. 2011;54(2):522–31. doi: 10.1002/hep.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15(8):1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Role of p38 MAPK in CYP2E1-dependent arachidonic acid toxicity. J Biol Chem. 2003;278(2):1115–24. doi: 10.1074/jbc.M207856200. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. Direct interaction of the Kruppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143(1):62–73. doi: 10.1210/endo.143.1.8590. [DOI] [PubMed] [Google Scholar]
- Zhao W, Goswami PC, Robbins ME. Radiation-induced up-regulation of Mmp2 involves increased mRNA stability, redox modulation, and MAPK activation. Radiat Res. 2004;161(4):418–29. doi: 10.1667/3155. [DOI] [PubMed] [Google Scholar]
- Zhenzhen Z, De’an T, Limin X, Wei Y, Min L. New Candidate Tumor-Suppressor Gene KLF6 and its Splice Variant KLF6 SV2 Counterbalancing Expression in Primary Hepatocarcinoma. Hepatogastroenterology. 2011;59(114) doi: 10.5754/hge11283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.