Abstract
Context:
Familial glucocorticoid deficiency (FGD) is a rare autosomal recessive disorder characterized by isolated cortisol deficiency. Mutations in the gene encoding the ACTH receptor (MC2R) account for 25% of cases. One significant feature is generalized skin hyperpigmentation, which is thought to be due to elevated ACTH acting on the melanocortin 1 receptor (MC1R).
Objective:
The aim of the study was to determine the cause of a nonhyperpigmented case of FGD.
Patients:
The patient presented at 4 yr of age with hypoglycemia after prolonged fasting during a respiratory tract infection. She had further hypoglycemic attacks and was diagnosed with isolated glucocorticoid deficiency at 6 yr of age. Her parents were consanguineous, and she had two unaffected sisters. Her physical examination was normal, except that her height and weight were greater than the 97th centile for a sex- and age-matched reference population. Interestingly, she had no hyperpigmentation despite very high ACTH levels.
Results:
Nucleotide sequence analysis revealed homozygous mutations c.478C>T in MC1R and c.455C>A in MC2R leading to R160W and T152K changes in the amino acid sequences, respectively. The R160W MC1R change has previously been implicated in a red hair/pale skin phenotype, and MC2R -T152K is trafficking defective. Both parents and two unaffected sisters were heterozygous for the MC1R mutation; additionally, one unaffected sister was heterozygous for the MC2R mutation, and the other was wild-type.
Conclusion:
We report an unusual case of FGD without hyperpigmentation due to coexistent MC1R/MC2R mutations. This case is important because it demonstrates for the first time that the assumption that the action of ACTH on MC1R causes skin hyperpigmentation is correct.
Familial glucocorticoid deficiency (FGD) is a rare autosomal recessive disorder in which cortisol and adrenal androgen secretions are deficient due to adrenal unresponsiveness to ACTH stimulation. The most significant phenotypic features are severe neonatal hypoglycemia, frequent childhood infection, and excessive generalized skin pigmentation related to elevated proopiomelanocortin (POMC) products (1). FGD can be caused by mutations in genes encoding the ACTH receptor [melanocortin 2 receptor (MC2R)] or its accessory protein (MRAP), and a similar clinical presentation has been observed in some patients with specific “mild” mutations in the steroidogenic acute regulatory protein (2–4). Most commonly, MC2R is mutated, and this is referred to as FGD type 1 (OMIM 202200).
MSH (melanotropin) and ACTH are products of the same gene, POMC, and they regulate pigmentation and adrenocortical function, respectively (5). Mutations in POMC result in a red hair phenotype with metabolic abnormalities, including adrenal insufficiency and obesity (6).
MC1R plays a central role in the regulation of skin pigmentation, is expressed in melanocytes, and binds α-MSH and ACTH with similar affinity. MC1R activation increases the ratio of black, strongly photoprotective eumelanins to reddish, poorly photoprotective pheomelanins (7). Several MC1R variants are associated with red hair/fair skin (7–9). Increased circulating ACTH acting through MC1R is believed to be the cause of the hyperpigmentation seen in primary adrenal failure. There are, however, a few reports of Addison's cases without hyperpigmentation (10–21). So far, no pathogenetic mechanism has been described to explain this phenomenon, although it has been noted that it is more frequently observed in fair-skinned individuals.
Here we report coexistent homozygous MC2R and MC1R mutations in the same individual, causing an unusual presentation of FGD without hyperpigmentation.
Patient and Methods
The patient presented at 4 yr of age with hypoglycemia during a respiratory tract infection after prolonged fasting. She had three further hypoglycemic attacks in the following 2 yr during infections. At the age of 6, during evaluation of her fifth hypoglycemic attack, she was diagnosed with hypocortisolemia with elevated ACTH levels. A standard ACTH stimulation test revealed a subnormal response (Table 1). Absence of salt wasting with normal plasma renin activity and without elevation of adrenal glucocorticoid precursors was consistent with isolated glucocorticoid deficiency (Table 1).
Table 1.
Biochemical and hormonal values at baseline and after a standard dose (250 μg) ACTH test
| Patient levels | Reference ranges | |
|---|---|---|
| ACTH | >1250 pg/ml (275 pmol/liter)b | <46 pg/ml (10.1 pmol/liter)b |
| Cortisol at diagnosis | 2.56 μg/dl (71.1 nmol/liter)a | 5–23 μg/dl (139–639 nmol/liter)a |
| Cortisol in standard dose ACTH stimulation test (250 μg) | 4.9 μg/dl (136.1 nmol/liter)b | 5–23 μg/dl (139–639 nmol/liter)b |
| 6.6 μg/dl (183.3 nmol/liter)c | >20 μg/dl (>550 nmol/liter)c | |
| DHEAS | <15 μg/dl (0.39 μmol/liter) | 2.3–15 μg/dl (0.06–0.39 μmol/liter) |
| Na and K | 136 and 3.14 mEq/liter | 134–145 and 3.5–5.3 mEq/liter |
| Aldosterone | 50 pg/ml (138.8 pmol/liter) | 10–180 pg/ml (27.7–500 pmol/liter) |
| Plasma renin activity | 2.5 ng/ml · h (3.2 pmol/ml · h) | 1–6.5 ng/ml · h (1.3–8.4 pmol/ml · h) |
The patient was found to have hypocortisolemia at the time of hypoglycemia, but ACTH levels were not measured at this time because no hyperpigmentation was present. DHEAS, Dehydroepiandrosterone sulfate. Conversion factors: DHEAS, 1 μg/dl = 38.46 μmol/liter; cortisol, 1 μg/dl = 0.036 nmol/liter; aldosterone, 1 pg/ml = 0.36 pmol/liter; ACTH, 1 pg/ml = 4.54 pmol/liter; plasma renin activity, 1 ng/ml · h = 0.77 pmol/ml · h.
At the time of hypoglycemia (glucose, 25 mg/dl);
before, and
after ACTH stimulation test.
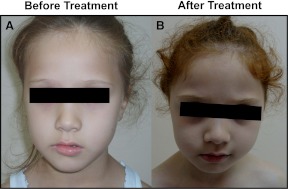
Her parents were consanguineous, and she had two unaffected sisters. Her physical examination was normal, except that her height and weight were above the 97th centile for height and weight of a sex- and age-matched reference population. It was noted that she had no hyperpigmentation, despite very high ACTH levels. Her eye color was bluish-gray, and skin and hair pigmentation was similar to unaffected family members. The family noted that her hair color was red during infancy and darkened as she got older. At follow-up, after replacement with hydrocortisone and reduction of serum ACTH levels, her hair had reverted to a reddish color (Fig. 1).
Fig. 1.
The patient before (A) and after treatment (B), showing the lack of hyperpigmentation and lightening of hair color on treatment.
Sequencing
Genomic DNA was extracted from whole blood samples after individuals gave informed consent. The sequences of the coding exons of MC1R and MC2R and their intron/exon junctions were determined by PCR and automated sequencing.
Results
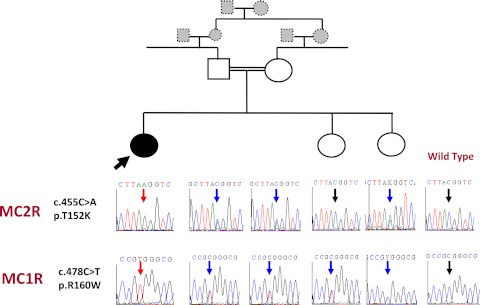
Nucleotide sequence analysis of MC2R revealed a homozygous mutation NC_000018.9:g.13885083C>A, c.455C>A (p.T152K), a loss-of function mutation previously shown to be trafficking defective (22) (Fig. 2). The parents and one unaffected sister were heterozygous for the mutation, and her other unaffected sister was wild-type. Hyperpigmentation is a classical clinical finding in FGD; to find the reason for this patient's lack of pigmentation, we sequenced MC1R. A homozygous NC_000016.9:g.89986144C>T, c.478C>T variation (p.R160W) was detected in MC1R in the proband. Her parents and sisters were heterozygous for the change (Fig. 2). This change, SNP ID rs1805008, has been linked to a red hair phenotype (8).
Fig. 2.
Pedigree and sequencing results of MC1R and MC2R for the patient and family members. The affected proband (black symbol and arrow) was homozygous for the indicated mutations. Unaffected parents and siblings (white symbols) were all heterozygous or wild-type for the mutations. Uncharacterized individuals are represented by gray symbols. Changes c.455C>A in MC2R (upper chromatograms) and c.478C>T in MC1R (lower chromatograms) are shown. Homozygous mutant and heterozygous and homozygous wild-type nucleotides are indicated by red, blue, and black arrows, respectively. Wild-type sequences from an unrelated control are indicated in the extreme right panels.
Discussion
Pigmentary skin changes are one of the most important clinical features and diagnostic clues in adrenal failure for clinicians. Here, we report a case of FGD without this clinical feature due to homozygous mutations in two unrelated genes, located on different chromosomes but belonging to the same family, the melanocortin receptors. The ligands of MC2R and MC1R are ACTH and α-MSH, respectively, both products of the same gene, POMC, which encodes a polypeptide hormone precursor that undergoes tissue-specific, posttranslational processing via cleavage by prohormone convertases. ACTH and α-MSH share the first 13 amino acids, and MC1R binds both peptides with similar affinity because of this structural similarity. Therefore, increased ACTH levels lead to hyperpigmentation in adrenal failure; in our case, skin pigmentation is absent due to a loss-of-function mutation in MC1R.
MC1R is a control point in the regulation of pigmentation; genetic variants are detected in over 80% of individuals with red hair and/or fair skin that tan poorly, but in fewer than 20% of individuals with brown or black hair (7). The R160W change reported in our case is one of most common changes reported in red-haired individuals (8) and reduces the cAMP response to α-MSH (23–25). Our patient had red hair when she was born, which darkened as she got older; after treatment and normalization of ACTH levels, her hair gradually lightened.
There are no reports of adrenal failure without pigmentation caused by AAAS, DAX-1, or SF-1 mutation, but some nonpigmented, so-called “white Addison's” cases have been reported (10–21). All such cases have been recorded in Europeans with fair skin, which suggests that MC1R variants could be implicated; in many instances, diagnosis was unexpected or delayed because of the absence of pigmentation. In only one patient was a mechanism for the absence of hyperpigmentation proposed; it was attributed to generalized vitiligo, with a high degree of melanosome degradation in secondary lysosomes being reported in a skin biopsy (15).
Here we report the first case of coexistent MC2R and MC1R homozygous mutations resulting in an unusual case of FGD1 without hyperpigmentation. The finding of a MC1R mutation may explain the absence of hyperpigmentation in this case. Additionally, MC1R variants could explain cases of white Addison's disease and might also handicap early phenotypic recognition of adrenal failure.
Acknowledgments
This work has been supported by the Medical Research Council UK (New Investigator Research Grant G0801265, to L.A.M.; and Clinical Research Training Fellowship Grant G0901980, to C.H.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page E802
- FGD
- Familial glucocorticoid deficiency
- MC2R
- melanocortin 2 receptor
- POMC
- proopiomelanocortin.
References
- 1. Chung TT, Chan LF, Metherell LA, Clark AJ. 2010. Phenotypic characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clin Endocrinol (Oxf) 72:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark AJ, McLoughlin L, Grossman A. 1993. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341:461–462 [DOI] [PubMed] [Google Scholar]
- 3. Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ. 2005. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- 4. Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ. 2009. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab 94:3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pritchard LE, White A. 2007. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 148:4201–4207 [DOI] [PubMed] [Google Scholar]
- 6. Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. 1998. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- 7. Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. 1995. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet 11:328–330 [DOI] [PubMed] [Google Scholar]
- 8. Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, Jacques JP, Rogers S, Turner R, Jackson IJ, Birch-Machin MA, Rees JL. 1998. Melanocortin 1 receptor variants in an Irish population. J Invest Derm 111:119–122 [DOI] [PubMed] [Google Scholar]
- 9. Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL. 2000. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet 9:2531–2537 [DOI] [PubMed] [Google Scholar]
- 10. Goodwin TJ, Kind PR, Bogomoletz VW. 1973. Addison's disease without pigmentation. Postgrad Med J 49:305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krause WKH. 2009. Cutaneous manifestations of endocrine diseases. Berlin, Heidelberg: Springer-Verlag; 45 [Google Scholar]
- 12. Dunlop D. 1963. Eighty-six cases of Addison's disease. Br Med J 2:887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fellows RE, Buchanan JR, Peterson RE, Stokes PE. 1962. Chronic primary adrenal insufficiency without hyperpigmentation. N Engl J Med 267:215–218 [Google Scholar]
- 14. Allison MF, Bailey IS, Curtin DC. 1964. Crisis following corticotrophin in Addison's disease without pigmentation. Postgrad Med J 40:26–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kendereski A, Micić D, Sumarac M, Zorić S, Macut D, Colić M, Skaro-Milić A, Bogdanović Z. 1999. White Addison's disease: what is the possible cause? J Endocrinol Invest 22:395–400 [DOI] [PubMed] [Google Scholar]
- 16. Runcie CJ, Semple CG, Slater SD. 1986. Addison's disease without pigmentation. Scott Med J 31:111–112 [DOI] [PubMed] [Google Scholar]
- 17. Barnett AH, Espiner EA, Donald RA. 1982. Patients presenting with Addison's disease need not be pigmented. Postgrad Med J 58:690–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heil G. 1981. Problems in the diagnosis of Addison's disease in the absence of hyperpigmentation. Z Gesamte Inn Med 36:333–334 [PubMed] [Google Scholar]
- 19. Hedinger C. 1950. Addison's disease without increase of pigmentation (so-called white Addison's disease). Schweiz Med Wochenschr 80:489–491 [PubMed] [Google Scholar]
- 20. Jores A, Tamm J. 1959. On a case of so-called “white” Addison's disease with Cushing-like habitus after substitution with prednisone. Acta Endocrinol (Copenh) 32:519–526 [PubMed] [Google Scholar]
- 21. Siebenlist D. 1995. “White Addison's disease” as an intensive care emergency. Dtsch Med Wochenschr 120:346–347 [PubMed] [Google Scholar]
- 22. Chung TT, Webb TR, Chan LF, Cooray SN, Metherell LA, King PJ, Chapple JP, Clark AJ. 2008. The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. J Clin Endocrinol Metab 93:4948–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott MC, Wakamatsu K, Ito S, Kadekaro AL, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing VJ, Abdel-Malek ZA. 2002. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci 115:2349–2355 [DOI] [PubMed] [Google Scholar]
- 24. Schiöth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL. 1999. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun 260:488–491 [DOI] [PubMed] [Google Scholar]
- 25. Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA. 2005. Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells. Peptides 26:1818–1824 [DOI] [PubMed] [Google Scholar]