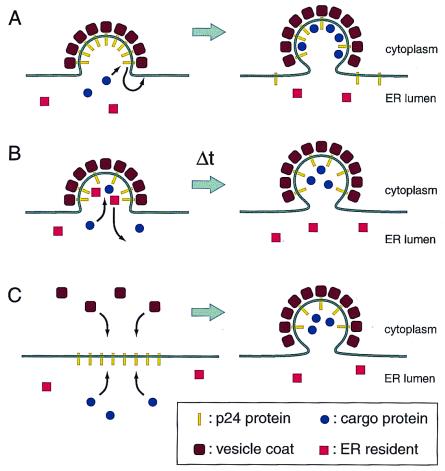
Figure 2.
Three speculative mechanisms for how p24 proteins might increase the fidelity of sorting by ER vesicles. (A) Cargo exclusion. As abundant constituents of the vesicle buds that interact with coat complexes, the p24 proteins may exclude proteins such as ER residents. Only cargo proteins that have high-affinity binding sites the interior surface of the vesicle would be able to displace a p24 protein and to occupy a site within the vesicle. (B) Time delay. Vesicle coat assembly may be intrinsically faster than sorting processes. The presence of p24 proteins in the membrane may force a delay in the time of budding, allowing sorting to reach completion. (C) Membrane segregation. p24 proteins may self-associate to create a specialized domain of the membrane. This domain would be expected to be a preferred site for coat assembly and may also serve as an attachment site for sorting factors in the lumen.