Abstract
Cadmium (Cd) in soil poses a major threat to plant growth and productivity. In the present experiment, we studied the effect of calcium (Ca2+) and/or potassium (K+) on the antioxidant system, accumulation of proline (Pro), malondialdehyde (MDA), and content of photosynthetic pigments, cadmium (Cd) and nutrients, i.e., Ca2+ and K+ in leaf of Vicia faba L. (cv. TARA) under Cd stress. Plants grown in the presence of Cd exhibited reduced growth traits [root length (RL) plant−1, shoot length (SL) plant−1, root fresh weight (RFW) plant−1, shoot fresh weight (SFW) plant−1, root dry weight (RDW) plant−1 and shoot dry weight (SDW) plant−1] and concentration of Ca2+, K+, Chlorophyll (Chl) a and Chl b content, except content of MDA, Cd and (Pro). The antioxidant enzymes [peroxidase (POD) and superoxide dismutase (SOD)] slightly increased as compared to control under Cd stress. However, a significant improvement was observed in all growth traits and content of Ca2+, K+, Chl a, Chl b, Pro and activity of antioxidant enzymes catalase (CAT), POD and SOD in plants subjected to Ca2+ and/or K+. The maximum alleviating effect was recorded in the plants grown in medium containing Ca2+ and K+ together. This study indicates that the application of Ca2+ and/or K+ had a significant and synergistic effect on plant growth. Also, application of Ca2+ and/or K+ was highly effective against the toxicity of Cd by improving activity of antioxidant enzymes and solute that led to the enhanced plant growth of faba bean plants.
Keywords: antioxidant, faba bean, heavy metal, nutrient content, photosynthetic pigments
1. Introduction
Increasing cadmium (Cd) in soil is an enormous constraint for agriculture worldwide. Cd is a highly toxic metal that causes deleterious effects on plants, and limits crop productivity [1]. Cd is commonly released into the arable soil from industries, energy, municipal sources and farming practices, and has been ranked No. 7 among the top 20 toxins [2]. Cd is easily taken up by plant roots and transported to the leaves through xylem [3]. Most plants are sensitive to low concentration of Cd, which disturb the physiological and molecular mechanisms by which plants carry out adaptive response to the environmental stresses. Therefore, it is important to understand the physiological mechanisms by which plants could perform normally under abiotic stress. Cd causes inhibition of physiological process such as photosynthesis, respiration, cell elongation, plant water relationship and assimilation of nitrogen, sulphur and phosphate, resulting in poor growth and development of plants [1,4]. Cd also disturbs the ion homeostasis in plants. The presence of Cd in soil perturbs the absorption of nutrients such as Ca, Mg, K, N, P, Fe, Mn, Cu, Zn and Ni [5–7]. However, the mechanisms involved in its toxicity as well as the cell response against the metal have not been well established [8,9].
Application of nutrients has become indispensable factor that has a broad influence on the growth and metabolism of plants under the condition of different soil environments [10]. One possible approach to minimize the Cd stress on plant productivity is through the addition of mineral nutrients, of which potassium (K+) has an outstanding role in plant growth and development. K+ is the most abundant cation in plants (up to 10% on dry weight basis) [11]. It is present in high concentration in cytosol and chloroplast, and activates many enzymes by stabilizing the pH between 7 and 8, by changing the enzymatic conformation, and also by binding the enzymes surface [12,13]. A large number of enzymes are either completely dependent on or stimulated by K+; more than 50 enzymes are activated by K+ [14]. It induces the cell elongation and maintains osmoregulation. K+-assimilation is an essential pathway for offsetting the Cd stress in plants by giving stimulatory influence of synthesis of protein, soluble carbohydrates and soluble nitrogen containing compounds (12), hence, these solutes may play a role in osmotic adjustment. Besides K+, calcium (Ca2+) has a significant role in alleviating the inhibitory effect of Cd on growth and physiological processes [15,16], and also increasing antioxidant enzyme activities, and in reducing lipid peroxidation of cell membranes [16,17]. Ca2+ plays an important role in heavy metal detoxification and in tolerance of plant to biotic and abiotic stress [18–20]. Ca2+ has also been shown to stabilize cell membrane surfaces, influence the pH of cells and prevent solute leakage from cytoplasm [21]. It is required for various structural roles in the cell wall and membranes; it is a counteraction for inorganic and organic anions in the vacuole, and the cytosolic. Ca2+ concentration is one of many cellular network parameters orchestrating complex cellular signaling coordinating responses to numerous developmental cues and environmental challenges [20,22,23].
Several studies have reported that the application of Ca2+ inhibits the K+ absorption or transportation in plants [24–26]. However, inhibitory effects of Ca2+ on K+ absorption are temporary and time-dependent because Ca2+ is needed to maintain the integrity of cellular membranes and of selective ion transport mechanisms by which K+ is actively absorbed [17,21,25,27,28]. Research has also shown that Ca2+ can significantly promote the absorption of K+ [25]. Moreover, some research has been done on the interaction between nutrients [29–31] and also on the influence of nutrients under heavy metals stress [32–35]. However, the mechanism involved in absorption and transport of heavy metals in plants is still unclear related to the soil environment [10]. Therefore, it is important to study the interactions between nutrients and heavy metals. However, scanty information is available on the efficacy of Ca2+ and K+ in offsetting the Cd stress in plants. Thus, we focused on Ca2+ and K+ as possible inducers in the tolerance of faba bean to Cd stress. The major objectives of our study were to determine the interactive efficiency of Ca2+ and K+ treatments in restoring the metabolic alterations resulting from Cd stress in faba bean.
2. Results
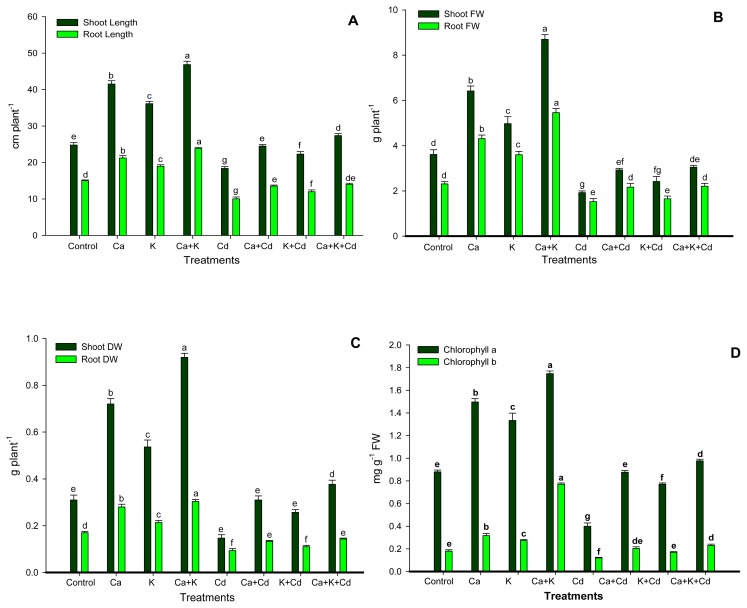
The significant changes of growth parameters in faba bean plants subjected to Ca2+ and/or K+ under Cd stress and normal conditions are shown in Figure 1A–C. Application of Ca2+ and K+ individually as well as in combination improved root length (RL) plant−1, shoot length (SL) plant−1, root fresh weight (RFW) plant−1, shoot fresh weight (SFW) plant−1, root dry weight (RDW) plant−1 and shoot dry weight (SDW) plant−1], as compared with the controls. However, combined application of Ca2+ and K+ showed more enhancing effect on all growth traits than individual treatment. Application of Cd inhibited all growth attributes of faba bean. However, the subsequent treatment of Cd-stressed plants with Ca2+ or K+ alone as well as in combination alleviated the adverse effect of Cd on faba bean and caused a considerable improvement of RL, SL, SFW, RFW, SDW, and RDW. The combined application of Ca2+ and K+ had maximum alleviating effect of Cd toxicity on all growth characteristics when compared with Ca2+ and K+ supplied alone. In contrast, combined application of Ca2+ and K+ showed parity with the application of Ca2+ alone for SFW and RFW.
Figure 1.
Ameliorating effect of calcium and potassium on shoot and root length (A), shoot fresh weight (FW) and root FW (B), shoot dry weight (DW) and root DW (C) and Chl a and Chl b (D) of faba bean plants under Cd stress. Bars followed by the same letters show no statistical difference at p < 0.05 (Duncan Multiple Range Test). Average of four determinations are presented with bars indicating SE.
Figure 1D reveals that application of Ca2+ and K+ alone as well as together significantly increased the chlorophyll (Chl) a and Chl b as compared to the control under normal conditions. The combined application of Ca2+ and K+ exhibited maximum value for both Chl a and Chl b when compared with individual application of Ca2+ and K+, under normal conditions. However, presence of Cd in growth medium suppressed both the photosynthetic pigments. Application of Ca2+ and/or K+ significantly enhanced Chl a and Chl b under stress. Moreover, application of Ca2+ and K+ together was found to be more effective in alleviating the adverse effect of Cd stress on Chl a and Chl b.
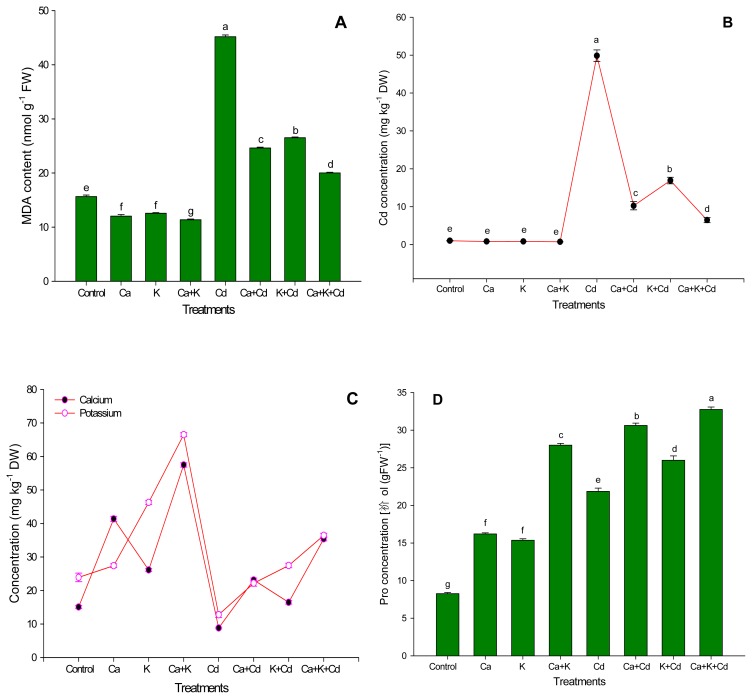
Figure 2A showed that Cd supplied in growth medium triggered accumulation of MDA, when compared with control. In contrast, under normal conditions, application of Ca2+ and/or K+ inhibited accumulation as compared to the control. However, application of Ca2+ and K+ alone as well as in combination arrested the ill effect of Cd up to a considerable limit by decreasing the accumulation of MDA in the leaf of faba bean plants. Under stress, the lowest accumulation of MDA was recorded in the plants that received Ca2+ and K+ together.
Figure 2.
Ameliorating effect of calcium and potassium on MDA content (A), Cd content (B), Ca2+ and K+ content (C) and Pro content (D) of faba bean plants under Cd stress. Bars followed by the same letters show no statistical difference at p < 0.05 (Duncan Multiple Range Test). Average of four determinations are presented with bars indicating SE.
Application of Cd increased the highest content of Cd in leaves as compared with control and other treatments (Figure 2B). However, under Cd stress, application of Ca2+ and K+ alone was found to be effective in suppressing leaf-Cd concentration, but maximum inhibition of Cd content was recorded in plants fed with the combined Ca2+ and K+ application. The presence of Cd in growth medium suppressed both the content of both nutrients in the leaf. However, application of Ca2+ and K+ alone as well as in combination increased leaf-Ca2+ and K+ content under normal as well as stress conditions. Application of Ca2+ and K+ individually was found to be effective in improving the content of leaf-Ca2+ and K+, but degree of efficiency of combined application of Ca2+ and K+ in alleviating the adverse effect of Cd stress on leaf-Ca2+ and K+ content was found to be maximum (Figure 2C).
It is evident from Figure 2D that proline (Pro) was higher in plant treated with combined application of Ca2+ and K+. Application of Ca2+ and K+ individually on unstressed plants could not bring about a significant change from control in the level of Pro. However, in association with Cd, they improved the quantity of Pro. Under stress conditions, application of Ca2+ gave maximum value for Pro when compared with K+ applied alone. The highest level of Pro was found in stressed plants, which were subjected to Ca2+ and K+ together.
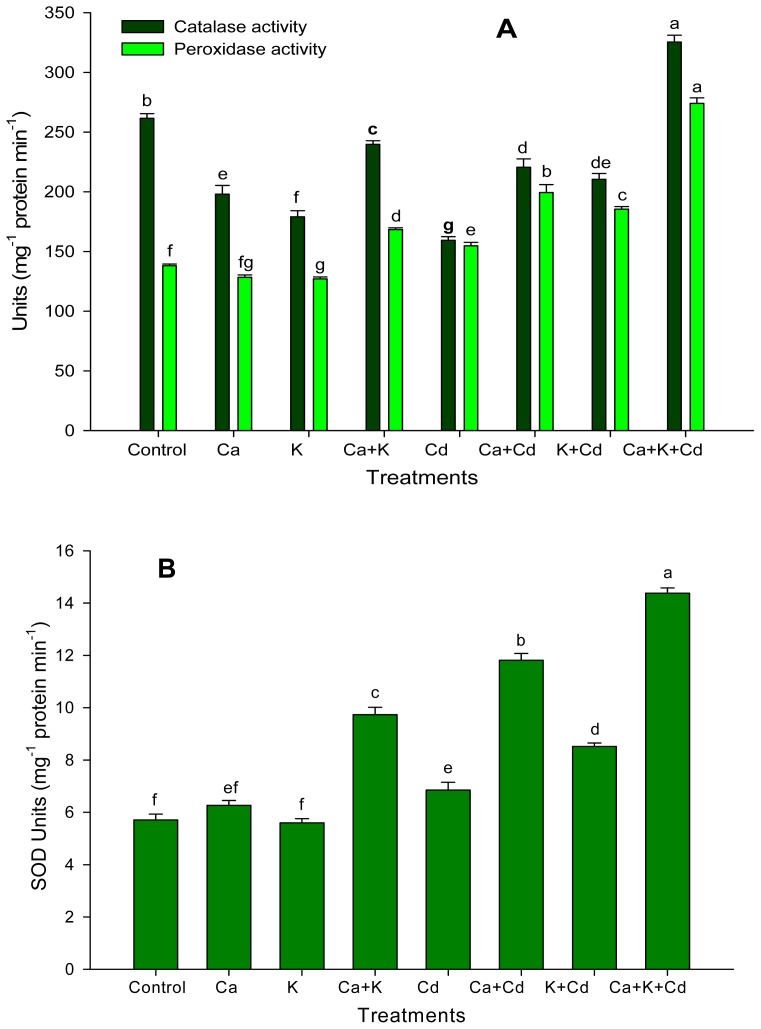
The changes in the activity of antioxidant enzymes, i.e., catalase (CAT), peroxidase (POD), and superoxide (SOD) in faba bean plants exposed to Ca2+ and/or K+ under both stress and non-stress conditions are shown in Figures 3A and 3B. Under non-stress conditions, application of Ca2+ and K+ individually decreased the activity of CAT and POD except SOD, but they gave a maximum value for these enzymes’ activity when they were applied together. Under Cd stress, application of Ca2+ individually gave maximum value for POD and SOD, but its effect was at par with K+ alone for CAT activity. However, the subsequent application of Cd-stressed plants with the combination of Ca2+ and K+ neutralized the adverse effect of Cd and resulted in considerable improvement in the activities of these three enzymes compared to their individual treatment (Figure 3).
Figure 3.
Ameliorating effect of calcium and potassium on the activity of (A) antioxidant enzymes catalase (CAT) and peroxidase (POD) and (B) superoxide dismutase (SOD) of faba bean plants under Cd stress. Bars followed by the same letters show no statistical difference at P<0.05 (Duncan Multiple Range Test). Average of four determinations are presented with bars indicating SE.
3. Discussion
It is well established that accumulation of Cd in plant tissues may cause a changes in various physiological processes resulting in poor growth, development and productivity of plants [1,9,36]. In the present experiment, plants subjected to Cd showed reduced growth in terms of RL, SL, RFW, SFW, RDW and SDW (Figures 1A–1C). The inhibition of growth traits may be due to the consequence of alteration of photosynthesis rate, and uptake and distribution of essential nutrients [37–39]. In the present study, application of Ca2+ and K+ alone as well as in combination was efficient to restore the altered plant growth induced by Cd toxicity in Vicia faba. The ameliorating response of both nutrients on plant growth is the consequence of cell elongation and cell division [21,40]; K+ acts as catalyst, even under adverse conditions, for many of the enzymatic processes that are necessary for plant growth and development [41]. Khan et al. [11] andSiddiqui et al. [35] reported that presence of Ca2+ in nutrient medium containing NaCl and Ni, promoted the plant growth. Thus, on the basis of the roles played by these nutrients, we could easily visualize their direct or indirect role in plant growth. This, in turn, could be responsible for the reversal of the altered plant growth induced by Cd stress; these improvements lead the plants with better adaptation under different adverse conditions. Thus, we may postulate that Ca2+ and K+ applied together in the present study were more effective in the restoration of altered plant growth characteristics (Figure 1).
The photosynthetic pigments are important macromolecules that are produced by plants, and play a vital role in photosynthesis, which is responsible for plant growth and dry matter production. In the present study, reduction of Chl a and b concentration induced by Cd stress might be due to the instability of proteins complex, destruction of chloroplast and photosynthetic apparatus [42], inhibition of photosynthetic electron transport chain [43] and the enzymes of Chl biosynthesis such as δ-aminolevulinic acid (ALA) synthase, ALA dehydratase, and porphobilinogenase [44]. Cadmium is involved in the inhibition of heme biosynthesis and chlorophyll synthesis by interacting with sulphydryl requiring enzymes involved in the pathway [45]. Interestingly, from this finding, it is very clear that the application of Ca2+ and/or K+ significantly enhance the concentration of these pigments under stress and non-stress conditions. Photosynthetic pigments could be increased due to the maximum presence of leaf-Ca2+ (Figure 2B) that could act as a secondary messenger for cytokinin action in promoting the Chl biosynthesis; Ca2+ could interact with light in the pathway of Chl synthesis [46]. Also, K+ plays an important role in the formation of photosynthetic pigment by preventing decomposition of newly formed Chl and ALA formation [47]. This result indicates that combined application of Ca2+ and K+ is associated with tolerance of faba bean plants to Cd stress by improving the biosynthesis of photosynthetic pigments.
In the present study, an elevated level of MDA concentration in leaves indicates that the plants were subjected to Cd stress induced lipid peroxidation, which resulted in membrane damage (Figure 2A). This is in accordance with other findings [48,49]. However, the plants that were subjected to Ca2+ and K+ alone as well as in combination, exhibited lowest values for MDA content (Figure 2A). This may be due to the antioxidant enzymes activity and Pro accumulation (Figures 2D and 3A, 3B) which has an adaptive significance, as they detoxify the oxygen free radicals and thus reduce the oxidative stress linked membrane deterioration under Cd stress. Also, the inhibition of MDA content may be due to the role of Ca2+ in controlling membrane structure and function by binding to phospholipids that stabilizes lipid bilayers and thus provides structural integrity to cellular membranes [21]. Furthermore, there is the possibility of the involvement of oxidative signal transduction concomitant with the regulation of antioxidant enzymes under stress [17,50]. Also, K+ plays a key role in reduction of ROS production by reducing activity of NAD(P)H oxidases and maintaining photosynthetic electron transport [41].
The accumulation of Cd in leaves of faba bean was high under Cd stress (Figure 2B). However, the accumulation of Cd was found to be lower in plants subjected to Ca2+ and K+ alone as well as in combination (Figure 2C). In this study, the highest accumulation of Ca2+ and K+ in leaf was recorded with the combined application of Ca2+ and K+, under stress and non-stress conditions. As evidenced in the present study, Cd caused a decrease in leaf-Ca2+ and K+ content in the plants (Figure 2C) which accords with the findings of Suzuki [15] and Umar et al. [51]. In Ca2+plus K+ treated plants, the increased accumulation of Ca2+ and K+ may be responsible for the inhibition of uptake and deleterious effects of Cd in Faba bean plants. The improvement of tolerance of plant to Cd stress may be due to the presence of Ca2+ that is responsible for active exclusion of toxic Cd by the formation and excretion of Cd/Ca2+ containing crystals through the head cells of trichomes [52]. It was also previously reported that Ca2+ was able to alleviate Cd toxicity, presumably through competition for metal ion influx [15]. Under abiotic stress, plants also require more K+ to maintain the photosynthesis because, during this process, reactive oxygen species (ROS) is generated [41]. The increased accumulation of Ca2+ and K+ with the application of Ca2+ and K+ alone as well as in combination have been major factors for increasing DW production and plant height because both are important components of many metabolically important compounds and play a vital role in various physiological processes [12].
Pro is not only a universal osmoprotectant, but also acts as an antioxidant as well as a source of energy; also it is considered to be an important biomolecule that has a protective role in tolerance of plant to abiotic stresses [16,35,53,54]. Cd stress leads to protein degradation through amino acid metabolism resulting in decreased plant growth [55]. In the present study, under Cd stress, accumulation of Pro was increased as compared to the control (Figure 2D) which is in accordance with the finding of Zhao [54]. However, application of Ca2+ and K+ individually as well as in combination was found to be effective to enhance further accumulation of Pro under stress and non-stress conditions (Figure 2D). The enhanced accumulation of Pro in leaves may represent a major biochemical adaptation, membrane stabilization and ROS scavenger [16,30,35,53,56]. Free Pro acts as an antioxidant in Cd-stressed cells and Pro levels are correlated with the GSH redox state and MDA levels in heavy metal-treated algae [57]. This result agrees with the finding of Siddiqui et al. [16], who reported that the production of Pro was higher in Ca2+-exposed plants under heavy metal stress.
The antioxidant system is one of the important defense mechanisms of plants, through which plants perform normally under different environmental conditions by scavenging ROS. In this experiment, antioxidant enzymes such as POD and SOD except CAT, increased slightly under Cd stress (Figures 3A, 3B). However, application of Ca2+ and K+ significantly enhanced the activity of all enzymes; the fact that the maximum increase in the activity of these enzymes was found in plants might be due to the combined application of Ca2+ and K+ (Figures 2A, 2B). The increased activity of antioxidant enzymes may be due to the active role of K+, which activates more than 50 enzymes [14]. Under stress, K+ plays important role in the synthesis of protein by participating in polypeptide synthesis in the ribosomes, since that process requires a high concentration of K+ [58]. Tripathi et al. [59] reported that proteins, such as thioredoxin, glutaredoxin, cyclophilin, among others, are known to facilitate the regeneration of the reduced (catalytically active) form of peroxiredoxins that play an important role in reducing the ROS formation in plants under biotic and abiotic stress. Also, Ca2+ acts as a secondary messenger of external stimuli, and stimulates calmodulin-like proteins that interact with Ca2+ ions. Changing their conformation in response to Ca2+ binding, calmodulin proteins regulate a variety of mechanisms, including ion transport, gene regulation, cell motility, growth, proliferation, apoptosis and stress tolerance that coordinate, at least in part, the plant response to Cd [60–62]. The Ca2+ might be responsible for decreased thiobarbutric acid and hydrogen peroxide content, as the unique importance of Ca2+ for stabilizing membranes is well established [21]. Khan et al. [17] and Siddiqui et al. [16] reported that application of Ca+2 was found to be effective in improving the tolerance of plant to abiotic stress by improving antioxidant systems. Thus, these results indicate that the improved activity of antioxidative enzymes due to the combined application of Ca2+ and K+ resulted in an increase in the capacity of various defense mechanisms and also improvement in various physiological and biochemical processes leading to the tolerance of the plant to Cd stress.
4. Experimental Section
4.1. Plant Cultures and Treatments
To meet the objectives mentioned in the introduction, the response of fabe bean (Vicia faba L.) to Ca2+ and K+ under Cd toxicity were studied by conducting a greenhouse pot experiment at the Department of Botany and Microbiology, King Saud University, Riyadh. Seeds of faba bean (cv. TARA) were obtained from a local market in Riyadh, Saudi Arabia. Healthy seeds were surface sterilized by using 1% sodium hypochlorite for 10 min then vigorously rinsed with sterilized double distilled water (DDW) before sowing. The seeds were sown in plastic pots (25 cm in diameter, 25 cm height) filled with perlite and supplied with Raukura’s nutrient solution [63]. The pots were arranged in a simple randomized design in the Glasshouse (Department of Botany and Microbiology, King Saud University, Riyadh, KSA) with a single factor and four replicates. The pots were covered with black plastic to reduce evaporation. One week after sowing, seedlings were thinned so that each pot contained healthy plants of uniform size. Pots were irrigated every two days with DDW (100 mL) to keep the perlite moist. Ca and K treatments were initiated 10 days after germination as follows: (i) Ca0+K0+Cd0 (control); (ii) Ca40 mM + K0 + Cd0; (iii) Ca0 + K6 mM + Cd0; (iv) Ca40 mM + K6 mM + Cd0; (v) Ca0 + K0 +Cd200 μM; (vi) Ca40 mM + K0 + Cd200 μM; (vii) Ca0 + K6 mM + Cd200 μM; (viii) Ca40 mM + K6 mM + Cd200 μM. The sources of Ca and K were calcium chloride and potassium chloride, respectively. Plants were sampled on the seventh day after treatment to assess their growth characteristics SL plant−1, RL plant−1, SFW plant−1, and RFW plant−1, SDW plant−1, RDW plant−1 and physiological attributes [Chl a and Chl b, concentrations of Ca2+, K+, Pro and MDA; and activity of CAT, POD and SOD].
4.2. Plant Growth Characteristics
Shoot length and RL were measured using a meter scale after removal from the pots. After recording FW with balance, plants were placed in a 60 °C oven for 48 h and then were weighed for DW.
4.3. Physiological and Biochemical Parameters
4.3.1. Chemical Content of Leaves
All the chemical reagents used in this procedure were of analytical grade. Absorbances were determined using a UV-VIS spectrophotometer, unless otherwise specified. Chlorophyll was extracted from fresh leaves using the DMSO method of Barnes et al. [64]. Chl a and Chl b concentrations were calculated based on the absorbance of the extract at 663.8 and 646.8 nm.
To determine Ca, K and Cd concentrations, we followed the digestion approach of Zheljazkov and Nielson [65] as modified by Hseu [66]. A leaf sample (0.5 g) was placed in a 250 mL digestion tube, and 10 mL of 2:1 concentrated nitric acid: perchloric acid was added. Samples were heated for 45 min at 90 °C for 2–3 h until a clear solution was obtained. At intervals, 5 mL of concentrated nitric acid: perchloric acid with hydrogen peroxide were added to the sample (at least three times), and the digestion continued until the volume was reduced to about 1 mL. The interior walls of the tube were washed down with a little DDW and the tubes were swirled throughout the digestion to keep the walls clean and prevent loss of the samples. After cooling, 5 mL of 1% HNO3 was added to each sample. Thereafter, the solution was filtered through Whatman No. 42 filter paper and <0.45 μm millipore filter paper. The filtrate was diluted to a total of 25 mL with distilled water. After dilution, the concentrations of Ca and K were determined by using with an atomic absorption spectrometer (Model 300, Perkin-Elmer, Waltham, MA, USA) and Cd was determined with the help of Inductively Coupled Plasma Optical Emission Spectroscope (Model iCAP6000, Thermo-Scientific, Thermo-Fisher Scientific, Waltham, MA, USA).
Proline concentration was determined spectrophotometrically by adopting the ninhydrin method of Bates et al. [67]. We first homogenized 300 mg of fresh leaf samples in sulphosalicylic acid, then added 2 mL each of acid ninhydrin and glacial acetic acid. The samples were heated at 100 °C. The mixture was extracted with toluene and the free toluene was quantified at 528 nm using L-proline as a standard.
Malondialdehyde (MDA) content was determined according to the method of Heath and Packer [68]. Leaves were weighed and homogenates containing 10% trichloroacetic acid (TCA) and 0.65% 2-thiobarbituric acid were heated at 95 °C for 60 min then cooled to room temperature and centrifuged at 10,000 × g for 10 min. The absorbance of the supernatant was read at 532 nm and 600 nm against a reagent blank.
4.3.2. Enzyme Activity
To determine the enzymatic activities of the antioxidant proteins, a crude enzyme extract was prepared by homogenizing 500 mg of leaf tissue in extraction buffer containing 0.5% Triton X-100 and 1% polyvinylpyrrolidone in 100 mM potassium phosphate buffer (pH 7.0) using a chilled mortar and pestle. The homogenate was centrifuged at 15,000 × g for 20 min at 4 °C. The supernatant was used for the enzymatic assays described below. All enzyme activities were expressed as units mg−1 protein min−1.
We used the method of Chance and Maehly [69] to determine POD (E.C. 1.11.1.7) activity by using 5 mL of an assay mixture containing phosphate buffer (pH 6.8), 50 M of pyrogallol, 50 mM of H2O2, and 1 mL of the enzyme extract diluted 20×. This was incubated for 5 min at 25 °C, after which the reaction was stopped by adding 0.5 mL of 5% (v/v) H2SO4. The amount of purpurogallin formed was determined by measuring absorbance at 420 nm. A unit of peroxidase activity was the amount of purpurogallin formed per mg protein per minute.
Aebi [70] method was used to measure CAT (EC 1.11.1.6) activity. The decomposition of H2O2 was monitored by the decrease in absorbance at 240 nm. For the assay, a 50 mM phosphate buffer (pH 7.8) and 10 mM H2O2 were used.
The activity of SOD (EC 1.15.1.1) was determined by measuring its ability to inhibit the photoreduction of nitro blue tetrazolium (NBT) according to the methods of Giannopolitis and Ries [71]. The reaction solution (3 mL) contained 50 μmol NBT, 1.3 μmol riboflavin, 13 mmol methionine, 75 nmol EDTA, 50 mmol phosphate buffer (pH 7.8), and 20 to 50 μL enzyme extract. The reaction solution was irradiated under a bank of fluorescent lights at 75 μmol·m−2·s−1 for 15 min. The absorbance at 560 nm was read against a blank (non-irradiated reaction solution). One unit of SOD activity was defined as the amount of enzyme that inhibited 50% of NBT photoreduction.
4.4. Statistical Analysis
Each pot was treated as one replicate and all the treatments were repeated four times. The data were analyzed statistically with SPSS-17 statistical software (SPSS Inc., Chicago, IL, USA). Means were statistically compared by Duncan’s Multiple Range Test (DMRT) at the p < 0.05% level.
5. Conclusions
In summary, results presented above confirm the importance of Ca2+ and/or K application in the detoxification of Cd toxicity. These results clearly show that application of Ca2+ and/or K+ decreased in MDA and Cd contents, and increased in photosynthetic pigment, Pro, antioxidant enzymes activity (CAT, POD and SOD) and accumulation of nutrients (Ca2+ and K+) may be responsible for better growth in terms of RL, SL, RFW, SFW that were responsible for enhanced dry matter production under Cd stress. Therefore, inclusion of Ca2+ and/or K+ in growth medium could be an adequate strategy to alleviate the harmful effects of heavy metal stress and to enhance plant metabolism to perform better under normal as well as different environmental conditions.
Acknowledgements
The financial support given by the Deanship of Scientific Research of King Saud University, Riyadh, KSA to the Research Group No. RGPVPP-153 is gratefully acknowledged.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Sanitá di Toppi L., Gabbrielli R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999;41:105–130. [Google Scholar]
- 2.Yang X.E., Long X.X., Ye H.B., He Z.L., Calvert D.V., Stoffella P.J. Cadmium tolerance and hyperaccumulation in a new Zn hyperaccumulating plant species (Sedum alfredii Hance) Plant Soil. 2004;259:181–189. [Google Scholar]
- 3.Leita L., de Nobili M., Cesco C., Mondini C. Analysis of intercellular cadmium forms in roots and leaves of bush bean. J. Plant Nutr. 1996;19:527–533. [Google Scholar]
- 4.Mishra S., Srivastava S., Tripathi R.D., Govindarajan R., Kuriakose S.V., Prasad M.N.V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol. Biochem. 2006;44:25–37. doi: 10.1016/j.plaphy.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Das P., Samantaray S., Rout G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997;98:29–36. doi: 10.1016/s0269-7491(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson D.T., Luttge U. Mineral nutrition: Divalent cations, transport and compartmentation. Progr. Bot. 1989;51:93–112. [Google Scholar]
- 7.Rivetta A., Negrini N., Cocucci M. Involvement of Ca2+-calmodulin in Cd2+ toxicity during the early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ. 1997;20:600–608. [Google Scholar]
- 8.Sandalio L.M., Dalurzo H.C., Gómez M., Romero-Puertas M.C., del Río L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Serrano M., Romero-Puertas M.C., Pazmiño D.M., Testillano P.S., Risueño M.C., del Río L.A., Sandalio L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150:229–243. doi: 10.1104/pp.108.131524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., Sun L., Sun T., Chao L., Guo G. Interaction between cadmium, lead and potassium fertilizer (K2SO4) in a soil-plant system. Environ. Geochem. Health. 2007;29:435–446. doi: 10.1007/s10653-007-9088-y. [DOI] [PubMed] [Google Scholar]
- 11.Leigh R.A., Jones R.G.W. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant-cell. New Phytol. 1984;97:1–13. [Google Scholar]
- 12.Marschner H. Mineral Nutrition of Higher Plants. 2nd ed. Academic Press; London, UK: 2002. [Google Scholar]
- 13.Mengel K. Potassium. In: Barker A.V., Pilbeam D.J., editors. Handbook of Plant Nutrition. CRC Press; Boca Raton, FL, USA: 2007. pp. 91–120. [Google Scholar]
- 14.Bhandal I.S., Malik C.P. Potassium estimation, uptake and its role in the physiology and metabolism of flowering plants. Int. Rev. Cytol. 1988;110:205–254. [Google Scholar]
- 15.Suzuki N. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotech. 2005;22:19–25. [Google Scholar]
- 16.Siddiqui M.H., Al-Whaibi M.H., Basalah M.O. Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma. 2011;248:503–511. doi: 10.1007/s00709-010-0197-6. [DOI] [PubMed] [Google Scholar]
- 17.Khan M.N., Siddiqui M.H., Mohammad F., Naeem M., Khan M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 2010;32:121–132. [Google Scholar]
- 18.Antosiewicz D.M., Hennig J. Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ. Pollut. 2004;129:237–245. doi: 10.1016/j.envpol.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Jáuregui-Zùñiga D., Ferrer M.A., Calderón A.A., Muñoz R., Moreno A. Heavy metal stress reduces the deposition of calcium oxalate crystals in leaves of Phaseolus vulgaris. J. Plant Physiol. 2005;162:1183–1187. doi: 10.1016/j.jplph.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Dayod M., Tyerman S.D., Leigh R.A., Gilliham M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma. 2010;247:215–231. doi: 10.1007/s00709-010-0182-0. [DOI] [PubMed] [Google Scholar]
- 21.Hirschi K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plieth C. Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- 23.White P.J., Broadley M.R. Calcium in plants. Ann. Bot. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handley R., Metwally A., Overstreet R. Effects of Ca upon metabolic and nonmetabolic uptake of Na and Rb by root segments of Zea mays. Plant Physiol. 1965;40:513–520. doi: 10.1104/pp.40.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elzam O.E., Hodges T.K. Calcium inhibition of potassium absorption in corn roots. Plant Physiol. 1967;42:1483–1488. doi: 10.1104/pp.42.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rains D.W., Epstein E. Sodium absorption by barley roots: Its mediation by mechanism 2 of alkali cation transport. Plant Physiol. 1967;42:319–323. doi: 10.1104/pp.42.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Steveninck R.F.M. The significance of calcium on the apparent permeability of cell membranes and the effects of substitution with other divalent ions. Physiol. Plant. 1965;18:54–69. [Google Scholar]
- 28.Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961;36:437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakobsen S.T. Interaction between plant Nutrients: III. Antagonism between potassium, magnesium and calcium. Acta Agric. Scand. Sect. B Soil Plant. 1993;43:1–5. [Google Scholar]
- 30.Siddiqui M.H., Khan M.N., Mohammad F., Khan M.M.A. Role of nitrogen and gibberellins (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008;194:214–224. [Google Scholar]
- 31.Malvi U.R. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci. 2011;24:106–109. [Google Scholar]
- 32.Oliver D.P., Schultz J.E., Tiller K.G., Merry R.H. The effect of crop rotations and tillage practices on cadmium concentration in wheat grain. Agric. Res. 1993;44:1221–1234. [Google Scholar]
- 33.Nie J.H., Liu X.M., Wang Q.R. Effects of nutrient elements on the lead uptake by hyperaccumulators. Ecol. Environ. 2004;13:306–309. [Google Scholar]
- 34.Tu C., Zheng C.R., Chen H.M. Effect of applying chemical fertilizers on forms of lead and cadmium in red soil. Chemosphere. 2000;41:133–138. doi: 10.1016/s0045-6535(99)00400-2. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui M.H., Mohammad F., Khan M.M.A., Al-Whaibi M.H. Cumulative effect of nitrogen and sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma. 2012;249:139–153. doi: 10.1007/s00709-011-0273-6. [DOI] [PubMed] [Google Scholar]
- 36.Perfus-Barbeoch L., Leonhardt N., Vavasseur A., Forestier C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–548. doi: 10.1046/j.1365-313x.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- 37.Benavides M.P., Gallego S.M., Tomaro M. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005;17:21–34. [Google Scholar]
- 38.Dong J., Wu F., Zhang G. Effect of cadmium on growth and photosynthesis of tomato seedlings. Zhejiang Univ. Sci. B. 2005;6:974–980. doi: 10.1631/jzus.2005.B0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurtyka R., Małkowski E., Kita A., Karcz W. Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Polish J. Environ. Stud. 2008;17:51–56. [Google Scholar]
- 40.Parmelee J.T., Beebe D.C. Decreased membrane permeability to potassium is responsible for the cell volume increase that drives lens fiber cell elongation. J. Cell Physiol. 1988;134:491–496. doi: 10.1002/jcp.1041340323. [DOI] [PubMed] [Google Scholar]
- 41.Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005;168:521–530. [Google Scholar]
- 42.Vassilev A., Iordanov I., Chakalova E., Kerin V. Effect of cadmium stress on growth and photosynthesis of young barley (H. vulgare L.) plants. 2. Structural and functional changes in the photosynthetic apparatus. Bujg. J. Plant Physiol. 1995;21:12–21. [Google Scholar]
- 43.Mohanty N., Vass I., Demeter S. Impairment of photosystem II activity at the level of secondary quinine acceptor in chloroplasts treated with cobalt, nickel and zinc ions. Physiol. Plant. 1989;76:386–390. [Google Scholar]
- 44.Shalygo N.V., Kolensikova N.V., Voronetskaya V.V., Averina N.G. Effects of Mn2+, Fe2+, Co2+ and Ni2+ on chlorophyll accumulation and early stages of chlorophyll formation of greening barley seedling. Russ. J. Plant Physiol. 1999;46:496–501. [Google Scholar]
- 45.Pandey S., Gupta K., Mukherjee A.K. Impact of cadmium and lead on Catharanthus roseus—A phytoremediation study. J. Environ. Biol. 2007;28:655–662. [PubMed] [Google Scholar]
- 46.Lechowski Z., Bialczyk J. Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biol. Plant. 1993;35:53–62. [Google Scholar]
- 47.Tanaka A., Tsuji H. Effects of calcium on chlorophyll synthesis and stability in the early phase of greening in cucumber cotyledons. Plant Physiol. 1980;65:1211–1215. doi: 10.1104/pp.65.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kórzyńska-Polit E., Drążkiewicz M., Krupa Z. Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiol. Plant. 2010;32:169–175. [Google Scholar]
- 49.Liu Z., Chen W., He X. Cadmium-induced changes in growth and antioxidative mechanisms of a medicine plant (Lonicera japonica Thunb.) J. Med. Plants Res. 2011;5:1411–1417. [Google Scholar]
- 50.McAinsh M.R., Clayton H., Mansfield T.A., Hetherington A.M. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 1996;111:1031–1042. doi: 10.1104/pp.111.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umar S., Diva I., Anjum N.A., Iqbal M. Potassium nutrition reduces cadmium accumulation and oxidative burst in mustard (Brassica campestris L.) Electron. Int. Fertil. Corresp. 2008;16:6–10. [Google Scholar]
- 52.Choi Y.E., Harada E., Wada M., Tsuboi H., Morita Y., Kusano T., Sano H. Detoxification of cadmium in tobacco plants: Formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta. 2001;213:45–50. doi: 10.1007/s004250000487. [DOI] [PubMed] [Google Scholar]
- 53.Matysik J., Alia B.B., Mohanty P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002;82:525–532. [Google Scholar]
- 54.Zhao Y. Cadmium accumulation and antioxidative defenses in leaves of Triticum aestivum L. and Zea mays L. African J. Biotech. 2011;10:2936–2943. [Google Scholar]
- 55.Dinakar N., Nagajyothi P.C., Suresh S., Udaykiran Y., Damodharam T. Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J. Environ. Sci. 2008;20:199–206. doi: 10.1016/s1001-0742(08)60032-7. [DOI] [PubMed] [Google Scholar]
- 56.Bandurska H. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol. Plant. 2001;23:483–490. [Google Scholar]
- 57.Siripornadulsil S., Traina S., Verma D.P.S., Sayre R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 2002;14:2837–2847. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones R.G., Pollard A. Proteins, Enzymes and Inorganic Ions. In: Läuchli A., Bieleski R.L., editors. Inorganic Plant Nutrition. Springer; New York, NY, USA: 1983. pp. 528–562. [Google Scholar]
- 59.Tripathi B.N., Bhatt I., Dietz K.J. Peroxiredoxins: A less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma. 2009;235:3–15. doi: 10.1007/s00709-009-0032-0. [DOI] [PubMed] [Google Scholar]
- 60.Yang T., Poovaiah B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003;8:505–512. doi: 10.1016/j.tplants.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Park H.Y., Kim S.A., Korlach J., Rhoades E., Kwok L.W., Zipfel W.R., Waxham M.N., Webb W.W., Pollack L. Conformational changes of calmodulin upon Ca2+ binding studied with a microfluidic mixer. Proc. Nat. Acad. Sci. USA. 2008;105:542–547. doi: 10.1073/pnas.0710810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DalCorso G., Farinati S., Furini A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2010;5:663–667. doi: 10.4161/psb.5.6.11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith G.S., Johnston C.M., Cornforth I.S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 1983;94:537–548. [Google Scholar]
- 64.Barnes J.D., Balaguer L., Manrique E., Elvira S., Davison A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992;32:85–100. [Google Scholar]
- 65.Zheljazkov V.D., Nielson N.E. Effect of heavy metals on peppermint and cornmint. Plant Soil. 1996;178:59–66. [Google Scholar]
- 66.Hseu Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004;95:53–59. doi: 10.1016/j.biortech.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- 68.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 69.Chance B., Maehly A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- 70.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 71.Giannopolitis C.N., Ries S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]