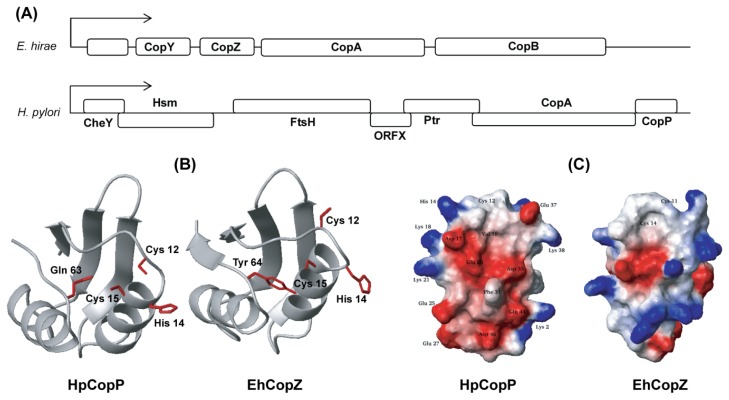
Figure 8.
Structural comparison between apo-HpCopP and apo-CopZ. (A) The composition of cop ORFs of H. pylori and E. hirae; (B) The orientation of the two cysteines and one histidine in the CXXC motif of HpCopP is compared with that of EhCopZ. The hydrophobic protection by Tyr 64 in loop V stabilizes the Cu(I)-coordination in EhCopZ. This residue is highly conserved in bacterial proteins, but is replaced with Gln 63 in HpCopP. The side-chain of Gln 63 is not fully exposed to the solvent and points toward the metal binding site in apo-HpCopP. The structures of EhCopZ (PDB ID: 1CPZ) were obtained from the PDB; (C) The electrostatic potential surfaces of HpCopP and EhCopZ are compared to each other. The positively and negatively charged residues are represented in blue and red, respectively.