Abstract
Upper gastrointestinal cancers commonly referred to as gastroesophageal carcinomas encompass cancers of the esophagus, stomach and gastroesophageal junction. Although the number of newly diagnosed cases of gastric cancer has decreased in the United States, the whole burden of upper gastrointestinal carcinomas on society remains significantly high, with only a small improvement in overall survival achieved over the past two decades. Traditionally, therapeutic agents used to treat gastroesophageal cancers have been platinums and fluoropyrimidines. Taxanes are di-terpenes produced by the plants of the genus Taxus (yews). As their name suggests, taxanes were first derived from natural sources, but now they are all synthesized artificially. Interfering with cellular microtubular function during cell division is the main mechanism of action for currently available taxanes. Since their introduction into therapeutic oncology, many different other taxane-derivatives have been manufactured and are being developed. Changing the formulation of the drug to improve delivery such as liposomal encapsulation, and target deliver with antibody-drug conjugation, as well as introducing new class of cytotoxic agents that can overcome taxane-resistance. The two most commonly used taxanes are paclitaxel and docetaxel. Taxane is a class of cytotoxic agents more commonly administered in patients with breast and lung cancers. However, the regulatory approval of docetaxel to treat patients with metastatic or advanced gastroesophageal cancers in 2006 established the role of taxanes in the management of upper gastroesophageal cancers. This paper will review the current data of taxanes in the management of patients with upper gastrointestinal cancers.
Keywords: Taxanes, gastric, esophageal, gastroesophageal junction, chemotherapy
Introduction
Upper gastrointestinal cancers, also refer to as gastroesophageal carcinomas (GECs) consist of cancers of the esophagus, stomach and gastroesophageal junction (GEJ). GECs are the fourth most frequently diagnosed cancer worldwide, and they are the second most common cause of cancer-related mortality (1). Since the late 1990s, the anatomic location of upper gastrointestinal carcinomas has shifted and this anatomic shift has varied geographically. In most Western countries, there has been an epidemiological shift: there has been a decrease in the incidence of GECs, but a steady increase in the incidence of cancers of the gastroesophageal junction (GEJ) (2),(3). Over the past 10-15 years, the anatomic primary site of upper gastrointestinal carcinomas in the West has shifted to the GEJ (2). An explanation for this phenomenon remains elusive, but speculation is that environmental factors common in Western countries, particularly the higher frequency of obesity, gastroesophageal reflux disease, and Barrett's esophagus, are the likely culprits. On the other hand patients in Eastern countries with a high prevalence of GECs, GECs are still primarily located in the distal gastrum and proximal esophagus (1). Complete surgical resection remains the only treatment option for long-term disease control and cure. However, because of the high rate of recurrence and the inaccuracy of clinical staging, surgery alone is associated with a 5-year overall survival (OS) rate of only 20-30% (4),(5). Multimodality therapy with concurrent chemotherapy, chemoradiotherapy (CRT), or both is commonly used to improve the duration of disease-free survival after complete surgical resection. Several recent randomized trials have shown improved survival outcomes when surgery is combined with another therapy (4)-(7). Unfortunately, more than 50% of newly diagnosed GECs are locally advanced (unresectable) or metastatic at the time of diagnosis. Among patients presenting with locoregional disease, less than 30% will have potentially resectable disease (8).
Randomized controlled trials have reported that a statistically significantly survival benefit can be attained with chemotherapy plus supportive care compared with supportive care alone, even in patients with locally advanced (unresectable) or metastatic GECs (9). However, patient selection is crucial to enhance the potential survival benefit in patients with advanced GECs. Antimetabolites, such as methotrexate, and alkylating agents, such as mitomycin, were a mainstay of early therapy for advanced GECs. While these agents remain important in the treatment of patients with other malignancies, their narrow therapeutic index of significant side effects and minimal improvement of outcomes, minimize any potential benefit for patients with advanced GECs. Until 2000, the only chemotherapeutic agents approved by the U.S. Food and Drug Administration (FDA) for the treatment of GECs included platinums (cisplatin, carboplatin), anthracyclines (doxorubicin, epirubicin), and pyrimidine analogs (5-fluorouracil [5-FU]). During that time span, treatment with chemotherapy resulted in only marginal survival improvement for patients with GECs (10). The combination of limited therapeutic options and narrow therapeutic indices of available agents resulted in disappointing treatment outcomes in patients with GECs. Until mass screening programs for GECs become available in Western countries, such as those already available in Japan, most GECs will continue to be diagnosed at more advanced stages. Overall, the prognosis of patients with GECs is poor, and it is particularly dismal for those with unresectable disease. To improve surgical outcomes or meaningful survival benefits, new effective cytotoxic or biologic targeted systemic therapies are needed for both resectable and unresectable or metastatic GECs.
Since 2006, the FDA has added a new indication for GECs to several cytotoxic agents. The main benefit of modifying older cytotoxic agents is an improved toxicity profile; examples of modified cytotoxic agents include oxaliplatin, which is a third-generation platinum, and capecitabine and S-1, which are modified or newer formulations of 5-FU. Prior to 2007, paclitaxel and docetaxel were already being used to treat patients with other solid tumor malignancies, but they did not have an FDA-approved indication for treating patients with GECs. In this paper, we will review the current roles taxanes in the management of GECs and discuss the future directions of their use.
Taxanes
Paclitaxel and docetaxel belong to the Taxane family because of their chemical structures contain a common three phenols ring. The clinical application of taxanes in the management of GECs predates their approval by the FDA for such an indication. It was not until 2006 that docetaxel received FDA approval for use as a first-line treatment in therapy-naïve patients with advanced GECs (11).
Taxanes are di-terpenes produced by the plants of the genus Taxus (yews). As their name suggests, taxanes were first derived from natural sources, but now they are all synthesized artificially. The two most commonly used taxanes are paclitaxel and docetaxel. Although all taxanes are currently used to treat patients with GECs, only docetaxel has an FDA-approved indication for use in combination with cisplatin and 5-FU to treat patients with GECs. Paclitaxel and docetaxel both have therapeutic indications for many solid tumor malignancies. However, only docetaxel has an FDA-approved indication for the treatment of advanced GECs. Paclitaxel has FDA-approved indications as a single agent for second-line therapy for metastatic ovarian cancer (12)-(16), for adjuvant treatment of node-positive breast cancer (17), and for second-line therapy for metastatic breast cancer (18), as well as for second-line therapy for Kaposi's sarcoma (19). In combination with cisplatin, paclitaxel is also indicated as first-line therapy for metastatic non-small cell lung (20) and ovarian (21),(22) cancers. Docetaxel was introduced at the end of the 1990s; it was first approved in 1996 for the treatment of refractory metastatic breast cancer (23)-(25). Additional FDA indications for early breast cancers (26),(27) and for advanced non-small cell lung cancer (28),(29), prostate cancer (30),(31), and metastatic head and neck cancers came later (32).
Paclitaxel
Paclitaxel was originally isolated from the bark of the Pacific yew tree, Taxus brevifolia. Its chemical structure was determined in 1971, and its mechanism of action was elucidated in 1979 (33). Paclitaxel is an anti-microtubule agent that irreversibly binds specifically to the subunit of the protein tubulin and promotes the assembly of microtubules. The stabilization of microtubules prevents normal mitotic spindle formation and function. This disruption of normal spindle function, which is the primary mechanism of action of paclitaxel (34),(35) ultimately results in chromosome breakage and inhibition of cell replication and migration. Therefore, paclitaxel inhibits cell replication by blocking cells in the late G2 and/or M phases of the cell cycle(35). Another important mechanism of action of paclitaxel includes induction of apoptosis via binding to and subsequently blocking the function of the apoptosis inhibitor-protein, bcl-2. Pharmacokinetics studies with paclitaxel have demonstrated that its distribution is a biphasic process, with values for α and β half-lives of approximately 20 minutes and 6 hours, respectively (33). True nonlinear pharmacokinetics may have important clinical implications, particularly in regards to dose modification, because a small increase in drug exposure and hence toxicity (33). More than 90% of the time, paclitaxel binds to plasma proteins. Approximately 71% of an administered dose of paclitaxel is excreted in the stool via the enterohepatic circulation (33). Renal clearance is minimal, accounting for 14% of the administered dose(33). In humans, paclitaxel is metabolized by cytochrome P-450 (CP-450) mixed-function oxidases. Specifically, either isoenzymes CYP2C8 and CYP3A4 of CP-450 will metabolize paclitaxel to hydroxylated 3' phydroxypaclitaxel (minor) and 6α-hydroxyplacitaxel (major), as well as to other forms of dihydroxylated metabolites. Paclitaxel is typically administered intravenously at a dose of 135-175 mg/m2 every 21 days (33),(36).
Docetaxel
While paclitaxel is a natural product, docetaxel is a semi-synthetic product. Docetaxel inhibits microtubule disassembly and promotes microtubule stabilization, leading to disruption of microtubule-mediated cellular function during cell division, cell cycle arrest at G2/M transition, and cell death (37). Like paclitaxel, docetaxel induces the activation of several molecular pathways leading to cellular apoptosis by disorganizing the microtubule structure (38). However, another proposed mechanism of action of docetaxel is related to its effect on phospholipase-D (PLD) (38). PLD has been implicated in several physiological processes, such as membrane trafficking, cytoskeletal reorganization, cell proliferation, differentiation, survival, and apoptosis (38). Pharmacokinetics studies with docetaxel have demonstrated a linear pharmacokinetic behavior with a 3-compartment model. Docetaxel binds to plasma proteins more than 95% of the time. Its metabolism also occurs via the CYP3A4 isoenzyme CP-450, and within 7 days of administration, 75% is eliminated in feces (38). Because most docetaxel is broken down in the liver, a reduced dose is recommended for patients with hepatic dysfunction, particularly those with elevated total bilirubin above the upper limit of normal (ULN) or alkaline phosphatase greater than 2.5 times ULN plus ALT and/or AST greater than 1.5 times ULN (38). Renal impairment or age greater than 75 years are an indication for docetaxel dose adjustment (38). Docetaxel is typically administered intravenously at a dose of 60-100 mg/m2 every 21 days (33),(39).
The most frequent dose-limiting toxicities (DLTs) of both paclitaxel and docetaxel include myelosuppression, hypersensitivity reactions, neuropathy, and musculoskeletal effects. Myelosuppression is both dose- and schedule-dependent, but it is not cumulative, where neutropenia is the principal DLT. The nadir of myelosuppression is usually on the 8th-10th day and complete bone marrow recovery is expected on the 15th-21th day (40). During its early development and in the initial phase II studies, docetaxel was administered at a dose of 100 mg/m2. In these early studies, neutropenia reached its nadir on the 8th day and resolved on the 15th-21st days of docetaxel infusion, and febrile neutropenia requiring hospitalization was observed in 10-14% of treated patients (38). Since its early development, docetaxel is now administered at a modified dose of 75 mg/m2. A significant reduction in febrile neutropenia frequency was observed with this dose (38).
Taxane hypersensitivity reactions can be categorized as type 1 (anaphylactoid) or type 2 (anaphylaxis). Symptoms of an anaphylactoid reaction include dyspnea, flushing, chest pain and tachycardia, where the cause is a surge of histamine release within 2-3 minutes after the administration of the drug. Anaphylaxis is more severe and can even be fatal; symptoms of anaphylaxis include hypotension, angioedema, and urticaria. Both types of reaction occur during the first two courses, and typically begin during the first 15 minutes of the infusion and resolve 15 minutes prior to the completion of the infusion. Along with antihistamine premedication, the administration of a prophylactic regimen consisting of 3-5 days of steroids beginning 1-2 days prior to treatment can reduce the frequency and severity of a hypersensitivity reaction (38),(40). Once patients have experienced either type of severe hypersensitivity reaction, the drug is further contraindicated. Fortunately, the incidence of anaphylaxis is low, occurring in only 2% of patients receiving paclitaxel and in 13% of patients receiving docetaxel.
Peripheral neuropathy resulting from both axonal degeneration and demyelination (40) is a DLT that is dose-dependent and cumulative. Mild symptoms relating to sensory loss usually improve or resolve completely within several months after discontinuation of therapy. Pre-existing neuropathies are not a contraindication to treatment. Central neurotoxicity may occur and may be severe especially with paclitaxel. Myalgia and/or arthralgia typically appear 2-3 days after drug administration, resolve within a few days, and are unrelated to dose (41),(42). Docetaxel-associated neuropathy occurs less frequently and with less severity than paclitaxel-associated neuropathy (42).
Reversible fluid retention syndrome (42),(43), which is characterized by edema and third-space fluid retention, is a unique side effect of docetaxel. Bowel wall edema and pleural and peritoneal fluid retention are common manifestations of this syndrome, which is caused by a docetaxel-induced increase in capillary permeability. The most serve end-organ complication of third-space fluid collection is heart failure. This severe complication can be ameliorated and prevented with prophylactic administration of corticosteroids, along with aggressive and early administration of diuretics (43).
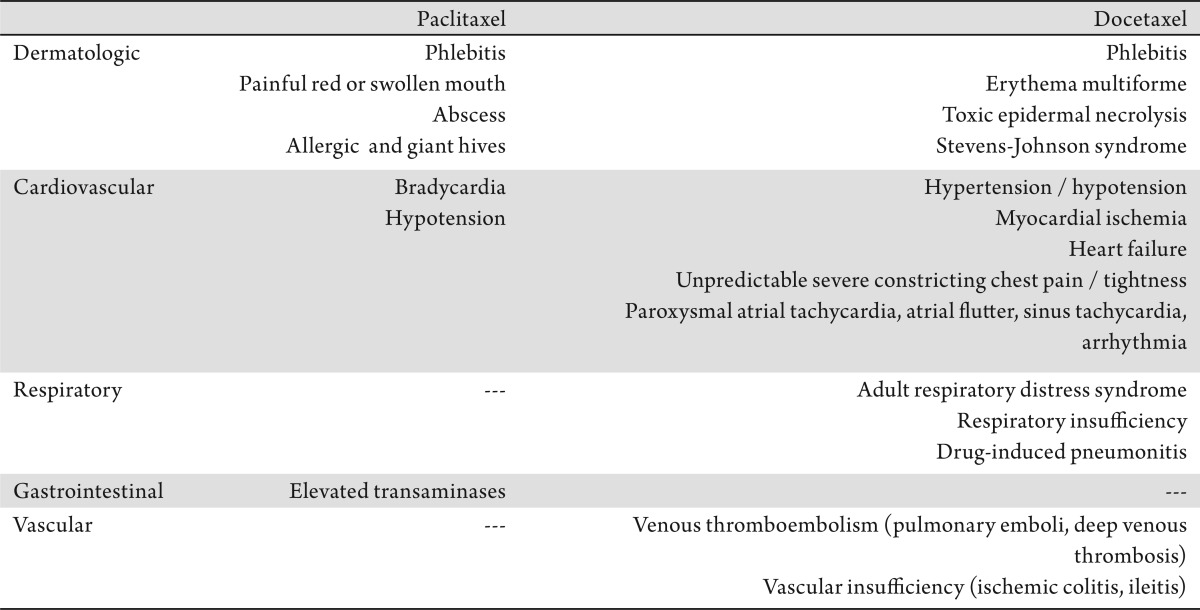
No less important, but less frequently reported, toxicities associated with taxanes include fatigue, mucositis, gastrointestinal symptoms, phlebitis, drug-induced adult respiratory distress syndrome (for docetaxel), and bradycardia plus swollen, red, painful mouth (for paclitaxel). Fatigue is observed in 58-67% of the patients treated with docetaxel, and it is occasionally severe enough to cause a modification in dose (33). Mucositis typically results from slow infusion, and it occurs more frequently in patients treated with docetaxel than with paclitaxel. Although less-severe gastrointestinal toxicityties, such as nausea, vomiting, and diarrhea, also occur more frequently with docetaxel, grade 3/4 gastrointestinal toxicities are uncommon (42). Table 1 summarizes the rare adverse effects associated taxanes.
Table 1. Rare side effects associated with taxanes.
Clinical use of taxanes in the treatment/management of advanced gastroesophageal cancers
For many solid tumors, tumor responses and survival outcomes are higher with CRT than with radiotherapy (RT) alone (44)-(49). For patients with solid tumors, CRT is used to palliate symptoms, treat definitively, and contribute significantly to multimodality therapy. Chemotherapeutic agents have been successfully used as radiosentisizers; platinums, fluoropyrimidines, and taxanes are the most commonly used chemotherapeutic agents.
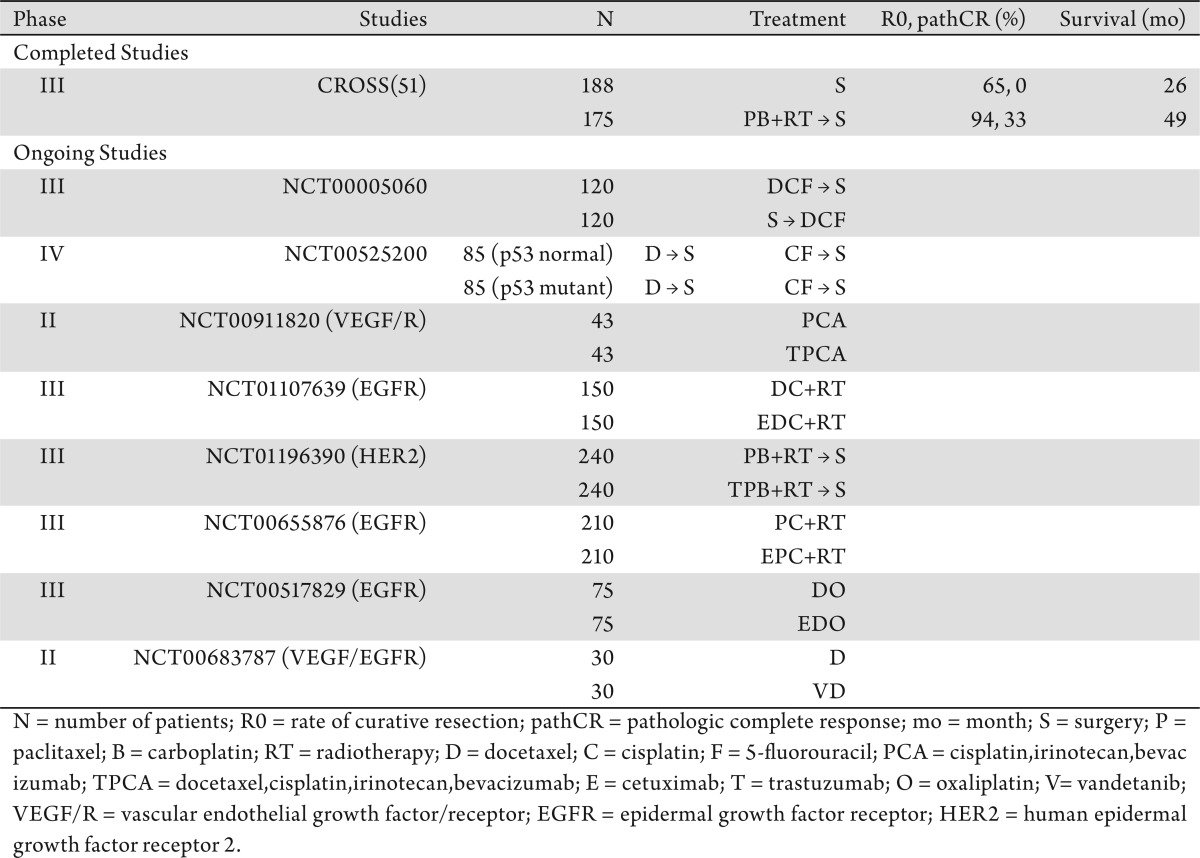
The results of the Radiation Therapy Oncology Group (RTOG) 85-01 trial (49) established that local disease control and survival outcome were both improved with CRT (RT combined with cisplatin and 5-FU) compared with RT alone. Therefore, most large randomized studies of CRT in GECs have been designed with either 5-FU, cisplatin, or both as radiosensitizers. Although taxanes are used as part of CRT for GECs, their use as radiosensitizers has been limited to phase II single-arm studies of patients with both resectable and locally advanced (unresectable) disease (50). Both paclitaxel and docetaxel are recognized to be potent radiosensitizers, and their effectiveness in GECs is demonstrated by the increased rates of curative resection, cancer down-staging and pathologic complete response (pCR) (51),(52). Many single-institutions, as well as cooperative, studies have suggested that taxane-based CRT is feasible, tolerable, and efficacious in patients with resectable GECs in either the preoperative or postoperative setting (51),(52). Preoperative paclitaxel-based CRT has demonstrated promising rates of pathologic responses, with observed pathCR rates of approximately 15-39% (53)-(57). Similar promising outcomes have been observed with preoperative docetaxel-based CRT (58)-(61). However, most of the efficacy data on taxane-based CRT come from small phase II studies because of what had been established as standard of care chemotherapeutic radiosensitizers by RTOG 85-01 (49). Results of the CROSS (51) study highlight taxane-based CRT and establish taxane-based CRT as a major contributor in a large phase III pivotal clinical trial of GECs. Patients with resectable esophageal cancer were randomly assigned to paclitaxel and carboplatin plus concurrent RT followed by surgery or to surgery alone. A total of 363 patients with resectable (T2/3 N0/1 M0) esophageal and GEJ cancers were enrolled. Preoperative CRT consisted of weekly administrations of paclitaxel 50 mg/m2 and carboplatin (AUC = 2) for 5 weeks and concurrent RT (41.4 Gy in 23 fractions, 5 days per week). Preoperative CRT did not affect surgery rates (86% vs. 90%) or in-hospital mortality rates (4% vs. 4%). However, R0 rates (92% vs. 65%) and pathCR rates (33% vs. 0%) improved after completing CRT. OS was significantly better (P = 0.011) in the group of patients treated with CRT (hazard ratio [HR] = 0.67; 95% confidence interval [95% CI], 0.50-0.92) likely establishing a new standard of care for patients with resectable GECs. The fact that the chemotherapy regimen used for CRT in the CROSS study did not include cisplatin and 5-FU is a significant departure from RTOG 85-01 (49).
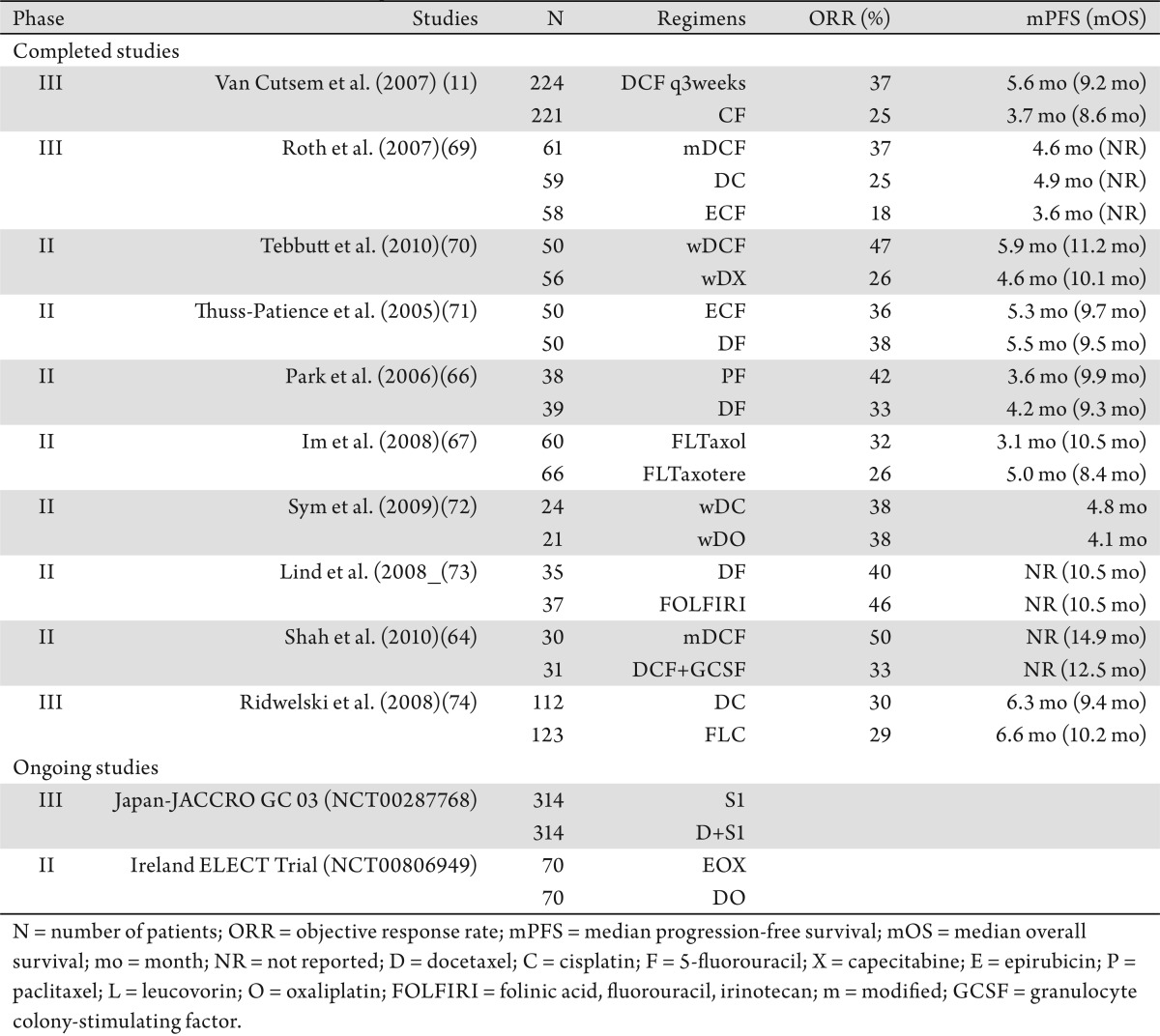
The cytotoxic activity and survival benefit of both paclitaxel and docetaxel have been demonstrated by many pivotal phase III clinical studies, with each positive study gaining these taxanes new FDA-approved indications for use in many different malignancies. V-325 (11) is a multi-institutional, international phase III study in which therapy-naïve patients with advanced or metastatic GC/GEJ cancers were randomized to receive either docetaxel (D) and cisplatin (C) plus 5-FU (DCF) or CF. Patients in the treat arm received DCF (docetaxel 75 mg/m2 day 1, cisplatin 75 mg/m2 day 1, plus infusional 5-FU 750 mg/m2/24 hours days 1-5) intravenously every 3 weeks. The primary end point was time to progression (TTP). A total of 457 patients (DCF 227, CF 230) were treated. Ajani et al. reported a more favorable TTP (5.6 vs. 3.7 months; HR = 1.47 [95% CI, 1.19-1.82]; P = 0.001) and OS (9.2 vs. 8.6 months; HR = 1.29 [95% CI, 1.0-1.6]; P = 0.02) in patients treated with DCF than with CF. Despite its promising results, V-325 (11) was severely criticized for its moderate toxicity; patients treated with DCF experienced more neutropenia (82% vs. 57%) and febrile neutropenia (29% vs. 12%) than those treated with CF. An ad hoc comparison of patients' benefits in terms of quality of life between the two arms concluded that DCF significantly prolonged time to definitive worsening of performance status versus CF (median, 6.1 vs. 4.8 months; HR = 1.38 [95% CI, 1.08-1.76]; P = 0.009) (62),(63). The results of this study led to FDA approval of docetaxel for gastric and GEJ cancers, but every-3-weeks DCF should be reserved for highly selected groups of patients.
Because docetaxel was found to be an active agent in GECs, many subsequent studies have offered modified and alternative docetaxel combinations in order to reduce toxicity and improve tolerance. In a randomized phase II study (64), Shah et al. observed moderate hematologic toxicity with DCF despite primary prophylaxis with growth colony-stimulating factor. Despite dose changes, modified DCF was noted to be much better tolerated while maintaining the same efficacy as its parent DCF.
In addition to dose and schedule modification of DCF regimens, many other docetaxel-based chemotherapy regimens have been evaluated. For instance, docetaxel has been combined with irinotecan, oxaliplatin, and S-1. S-1 is not currently available outside of clinical trials in the United States. The use of S-1 in advanced GECs in Western countries had been tempered by the negative results of the FLAGS (First Line therapy in Advanced Gastric cancer Study) study (65), comparing cisplatin plus 5-FU to cisplatin plus S-1.
Selecting between paclitaxel and docetaxel remains an art rather than science. Though commonly practiced, there are no convincing data in the medical literature on GEC to support the interchangeability between docetaxel and paclitaxel. In two randomized phase II studies (66),(67) from Asia comparing 5-FU combined with either paclitaxel or docetaxel, no statistically significant difference in therapeutic efficacy or survival outcomes was observed. It remains unclear if there is a significant difference between DCF (11) and ECF (68) or other standard regimens, or between docetaxel triplet and doublets. Table 2 summarizes selected randomized phase II or III studies with taxane-based chemotherapy regimens as first-line therapy for metastatic GECs.
Table 2. Taxane-based chemotherapy regimens: comparative phase II/III.
Conclusion and future direction
Taxanes are a class of cytotoxic agents commonly administered in patients with breast and lung cancers. Both paclitaxel and docetaxel, two commonly used taxanes, have many indications as both single agents as well as in combination therapy for many solid tumors. They have also been shown to contribute significantly to the management of patients with both localized and advanced GECs. Direct evidence for their use in the management of GECs is derived from the results of several phase II studies. Phase III studies with taxanes in GECs are limited. V-325 (11) and CROSS (51) are pivotal studies that not only changed how we treat GECs, but also validated the role of taxanes in the management of GECs. The V-325 (11) study is a pivotal randomized study that demonstrated that docetaxel-based chemotherapy improved TTP and OS in patients with advanced GEC. The CROSS (51) study demonstrated improvements in surgical outcomes and survival in patients treated with preoperative CRT with paclitaxel and carboplatin. Tables 2 and 3 summarize completed and ongoing clinical trials with taxanes-base chemotherapy, administered either alone or combined with targeted therapy.
Table 3. Combination taxane-based + targeted therapy.
The future development of taxanes for use in GEC will require establishing optimal taxane-based chemotherapy regimens to further develop with targeted therapy, evaluating possible ways of overcoming mechanisms of resistance to taxanes, and identifying molecular biomarkers that are predictive of response. This effort will require the collaborative efforts of many scientific disciplines.
Footnotes
No potential conflict of interest.
Reference
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–53. [PubMed] [Google Scholar]
- 3.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14:Sii31–6. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 6.Boige V, Pignon JP, Saint-Aubert B, Lasser P, Conroy T, Bouché O, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial [abstract] J Clin Oncol. 2007;s25:4510. [Google Scholar]
- 7.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 8.Varadhachary G, Ajani JA. Preoperative and adjuvant therapies for upper gastrointestinal cancers. Expert Rev Anticancer Ther. 2005;5:719–25. doi: 10.1586/14737140.5.4.719. [DOI] [PubMed] [Google Scholar]
- 9.Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–91. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varadhachary G, Ajani JA. Gastric cancer. Clin Adv Hematol Oncol. 2005;3:118–24. [PubMed] [Google Scholar]
- 11.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 12.Cantù MG, Buda A, Parma G, Rossi R, Floriani I, Bonazzi C, et al. Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol. 2002;20:1232–7. doi: 10.1200/JCO.2002.20.5.1232. [DOI] [PubMed] [Google Scholar]
- 13.Paclitaxel and docetaxel in breast and ovarian cancer. Drug Ther Bull. 1997;35:43–6. [PubMed] [Google Scholar]
- 14.Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME, et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol. 1994;12:2654–66. doi: 10.1200/JCO.1994.12.12.2654. [DOI] [PubMed] [Google Scholar]
- 15.Gore ME, Preston N, A'Hern RP, Hill C, Mitchell P, Chang J, et al. Platinum-Taxol non-cross resistance in epithelial ovarian cancer. Br J Cancer. 1995;71:1308–10. doi: 10.1038/bjc.1995.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, et al. Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103. J Clin Oncol. 1993;11:2405–10. doi: 10.1200/JCO.1993.11.12.2405. [DOI] [PubMed] [Google Scholar]
- 17.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop JF, Dewar J, Toner GC, Smith J, Tattersall MH, Olver IN, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999;17:2355–64. doi: 10.1200/JCO.1999.17.8.2355. [DOI] [PubMed] [Google Scholar]
- 19.Saville MW, Lietzau J, Pluda JM, Feuerstein I, Odom J, Wilson WH, et al. Treatment of HIV-associated Kaposi's sarcoma with paclitaxel. Lancet. 1995;346:26–8. doi: 10.1016/s0140-6736(95)92654-2. [DOI] [PubMed] [Google Scholar]
- 20.Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–31. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 21.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 22.Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 23.Nabholtz JM, Senn HJ, Bezwoda WR, Melnychuk D, Deschênes L, Douma J, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol. 1999;17:1413–24. doi: 10.1200/JCO.1999.17.5.1413. [DOI] [PubMed] [Google Scholar]
- 24.Chan S, Friedrichs K, Noel D, Pintér T, Van Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1999;17:2341–54. doi: 10.1200/JCO.1999.17.8.2341. [DOI] [PubMed] [Google Scholar]
- 25.Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, et al. Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol. 2006;24:4963–70. doi: 10.1200/JCO.2005.05.0294. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 27.Hurley J, Doliny P, Reis I, Silva O, Gomez-Fernandez C, Velez P, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24:1831–8. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 29.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 30.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 31.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 32.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs. 1998;55:5–30. doi: 10.2165/00003495-199855010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Spencer CM, Faulds D. Paclitaxel. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 1994;48:794–847. doi: 10.2165/00003495-199448050-00009. [DOI] [PubMed] [Google Scholar]
- 35.Rowinsky EK. Clinical pharmacology of Taxol. J Natl Cancer Inst Monogr. 1993;(15):25–37. [PubMed] [Google Scholar]
- 36.Ajani JA, Ilson DH, Kelsen DP. The activity of paclitaxel in gastrointestinal tumors. Semin Oncol. 1995;22:s46–50; discussion 1-3. [PubMed] [Google Scholar]
- 37.Tanaka M, Obata T, Sasaki T. Evaluation of antitumour effects of docetaxel (Taxotere) on human gastric cancers in vitro and in vivo. Eur J Cancer. 1996;32A(2):226–30. doi: 10.1016/0959-8049(95)00500-5. [DOI] [PubMed] [Google Scholar]
- 38.Deeks ED, Scott LJ. Docetaxel: in gastric cancer. Drugs. 2007;67:1893–901. doi: 10.2165/00003495-200767130-00007. [DOI] [PubMed] [Google Scholar]
- 39.Pazdur R, Lassere Y, Soh LT, Ajani JA, Bready B, Soo E, et al. Phase II trial of docetaxel (Taxotere) in metastatic colorectal carcinoma. Ann Oncol. 1994;5:468–70. doi: 10.1093/oxfordjournals.annonc.a058883. [DOI] [PubMed] [Google Scholar]
- 40.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:s1–15. [PubMed] [Google Scholar]
- 41.Baker J, Ajani J, Scotté F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009;13:49–59. doi: 10.1016/j.ejon.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Markman M. Management of toxicities associated with the administration of taxanes. Expert Opin Drug Saf. 2003;2:141–6. doi: 10.1517/14740338.2.2.141. [DOI] [PubMed] [Google Scholar]
- 43.Piccart MJ, Klijn J, Paridaens R, Nooij M, Mauriac L, Coleman R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol. 1997;15:3149–55. doi: 10.1200/JCO.1997.15.9.3149. [DOI] [PubMed] [Google Scholar]
- 44.Morton RF, Jett JR, McGinnis WL, Earle JD, Therneau TM, Krook JE, et al. Thoracic radiation therapy alone compared with combined chemoradiotherapy for locally unresectable non-small cell lung cancer. A randomized, phase III trial. Ann Intern Med. 1991;115:681–6. doi: 10.7326/0003-4819-115-9-681. [DOI] [PubMed] [Google Scholar]
- 45.Adelstein DJ, Saxton JP, Lavertu P, Tuason L, Wood BG, Wanamaker JR, et al. A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer: preliminary results. Head Neck. 1997;19:567–75. doi: 10.1002/(sici)1097-0347(199710)19:7<567::aid-hed2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 47.Kim TY, Yang SH, Lee SH, Park YS, Im YH, Kang WK, et al. A phase III randomized trial of combined chemoradiotherapy versus radiotherapy alone in locally advanced non-small-cell lung cancer. Am J Clin Oncol. 2002;25:238–43. doi: 10.1097/00000421-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–7. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 49.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 50.Ajani JA. Chemotherapy for advanced gastric or gastroesophageal cancer: defining the contributions of docetaxel. Expert Opin Pharmacother. 2006;7:1627–31. doi: 10.1517/14656566.7.12.1627. [DOI] [PubMed] [Google Scholar]
- 51.van der Gaast A, van Hagen PM, Hulshof M, Richel D, van Berge Henegouwen MI, Nieuwenhuijzen GA, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study [abstract] J Clin Oncol. 2010;s28:4004. [Google Scholar]
- 52.Ajani J. Therapy of localized esophageal cancer: it is time to reengineer our investigative strategies. Onkologie. 2008;31:360–1. doi: 10.1159/000137836. [DOI] [PubMed] [Google Scholar]
- 53.Ajani JA, Komaki R, Putnam JB, Walsh G, Nesbitt J, Pisters PW, et al. A three-step strategy of induction chemotherapy then chemoradiation followed by surgery in patients with potentially resectable carcinoma of the esophagus or gastroesophageal junction. Cancer. 2001;92:279–86. doi: 10.1002/1097-0142(20010715)92:2<279::aid-cncr1320>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 54.Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–44. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 55.Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–8. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 56.Kelsey CR, Chino JP, Willett CG, Clough RW, Hurwitz HI, Morse MA, et al. Paclitaxel-based chemoradiotherapy in the treatment of patients with operable esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:770–6. doi: 10.1016/j.ijrobp.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz GK, Winter K, Minsky BD, Crane C, Thomson PJ, Anne P, et al. Randomized phase II trial evaluating two paclitaxel and cisplatin-containing chemoradiation regimens as adjuvant therapy in resected gastric cancer (RTOG-0114) J Clin Oncol. 2009;27:1956–62. doi: 10.1200/JCO.2008.20.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choong NW, Mauer AM, Haraf DC, Ferguson MK, Sandler AB, Kesler KA, et al. Long-term outcome of a phase II study of docetaxel-based multimodality chemoradiotherapy for locally advanced carcinoma of the esophagus or gastroesophageal junction. Med Oncol. 2010;Aug 21 doi: 10.1007/s12032-010-9658-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Spigel DR, Greco FA, Meluch AA, Lane CM, Farley C, Gray JR, et al. Phase I/II trial of preoperative oxaliplatin, docetaxel, and capecitabine with concurrent radiation therapy in localized carcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2010;28:2213–9. doi: 10.1200/JCO.2009.24.8773. [DOI] [PubMed] [Google Scholar]
- 60.fKambysellis MP, Ho KF, Craddock EM, Piano F, Parisi M, Cohen J. Pattern of ecological shifts in the diversification of Hawaiian Drosophila inferred from a molecular phylogeny. Curr Biol. 1995;5:1129–39. doi: 10.1016/s0960-9822(95)00229-6. [DOI] [PubMed] [Google Scholar]
- 61.Ajani JA, Correa AM, Walsh GL, Komaki R, Lee JH, Vaporciyan AA, et al. Trimodality therapy without a platinum compound for localized carcinoma of the esophagus and gastroesophageal junction. Cancer. 2010;116:1656–63. doi: 10.1002/cncr.24935. [DOI] [PubMed] [Google Scholar]
- 62.Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3210–6. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 63.Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205–9. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 64.Shah M, Shibata S, Stoller RG, Kemeny M, Ritch PS, Krishnamurthi SS, et al. Random assignment multicenter phase II study of modified docetaxel, cisplatin, fluorouracil (mDCF) versus DCF with growth factor support (GCSF) in metastatic gastroesophageal adenocarcinoma (GE) [abstract] J Clin Oncol. 2010;s28:4014. [Google Scholar]
- 65.Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–53. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 66.Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006;17:225–9. doi: 10.1097/00001813-200602000-00015. [DOI] [PubMed] [Google Scholar]
- 67.Im CK, Jung SE, Rha SY, Ahn J, Shin S, Noh S, et al. Comparison of taxane-based (docetaxel or paclitaxel) regimens combined with 5-fluorouracil continuous infusion and low dose leucovorin for advanced gastric carcinoma: Analysis of two phase II trials [abstract] J Clin Oncol. 2008;s26:15679. [Google Scholar]
- 68.Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 69.Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217–23. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- 70.Tebbutt NC, Cummins MM, Sourjina T, Strickland A, Van Hazel G, Ganju V, et al. Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. Br J Cancer. 2010;102:475–81. doi: 10.1038/sj.bjc.6605522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thuss-Patience PC, Kretzschmar A, Repp M, Kingreen D, Hennesser D, Micheel S, et al. Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. J Clin Oncol. 2005;23:494–501. doi: 10.1200/JCO.2005.02.163. [DOI] [PubMed] [Google Scholar]
- 72.Sym SJ, Park S, Kwon KY, Jung I, Park J, Cho E, et al. A randomized phase II trial of weekly docetaxel plus either cisplatin or oxaliplatin in patients with previously untreated advanced gastric cancer: Preliminary results [abstract] J Clin Oncol. 2009 Abstr 92. [Google Scholar]
- 73.Lind PA, Gubanski M, Johnson AE, Fernebro E, Flygare P, Karlberg I, et al. Final results of the randomized phase II study of sequential docetaxel and irinotecan with infusion 5-fluorouracil/folinic acid in patients with advanced gastric cancer - GA-TAC [abstract] J Clin Oncol. 2008;s26:4579. [Google Scholar]
- 74.Ridwelski K, Fahlke J, Kettner E, Schmidt C, Keilholz U, Quietzsch D, et al. Docetaxel-cisplatin (DC) versus 5-fluorouracil-leucovorin-cisplatin (FLC) as first-line treatment for locally advanced or metastatic gastric cancer: Preliminary results of a phase III study [abstract] J Clin Oncol. 2008;s26:4512. [Google Scholar]