Abstract
Purpose
To prospectively examine alterations in working memory (WM) –associated brain activation related to breast cancer and treatment by using functional magnetic resonance imaging.
Patients and Methods
Patients treated with chemotherapy (CTx+; n = 16) or without chemotherapy (CTx−; n = 12) and healthy controls (n = 15) were scanned during an n-back task at baseline (after surgery but before radiation, chemotherapy, and/or antiestrogen treatment), 1 month after completion of chemotherapy (M1), and 1 year later (Y1), or at yoked intervals for CTx− and controls. SPM5 was used for all image analyses, which included cross-sectional between-group and group-by-time interaction and longitudinal within-group analyses, all using a statistical threshold of 0.001.
Results
At baseline, patients with cancer showed increased bifrontal and decreased left parietal activation compared with controls. At M1, both cancer groups showed decreased frontal hyperactivation compared with controls, with increased hyperactivation at Y1. These cross-sectional findings were confirmed by group-by-time interaction analyses, which showed frontal activation decreases from baseline to M1 in patients compared with controls. Within-group analyses showed different patterns of longitudinal activation change by treatment group (CTx+ or CTx−), with prominent alterations in the frontal lobes bilaterally.
Conclusion
Significant frontal lobe hyperactivation to support WM was found in patients with breast cancer. Superimposed on this background, patients showed decreased frontal activation at M1, with partial return to the previously abnormal baseline at Y1. These functional changes correspond to frontal lobe regions where we previously reported structural changes in this cohort and provide prospective, longitudinal data that further elucidate mechanisms underlying cognitive effects related to breast cancer and its treatment.
INTRODUCTION
Cognitive impairment related to breast cancer (BC) and its treatment has become an important area of study. A growing body of evidence demonstrates that a subgroup of patients with BC have pretreatment cognitive deficits,1–5 suggesting that aspects of cancer pathogenesis or host factors (potentially including variables related to BC surgery) cause cognitive changes or that there are common risk factors for the development of cancer and cognitive changes.6 Prospective studies have shown cognitive changes differentially attributable to chemotherapy, radiation, and antiestrogen treatment,7–11 highlighting the need for better understanding of potentially additive and dissociable effects of cancer treatments and the disease process on cognition in vulnerable individuals.12
Structural and functional neuroimaging allow examination of the neural substrates of cancer- and treatment-related cognitive changes, which currently are poorly understood. We recently reported findings from the first prospective, longitudinal magnetic resonance imaging (MRI) study to examine changes in brain gray matter density (GMD) after treatment for BC.13 By using voxel-based morphometry, we demonstrated GMD decreases in bilateral frontal, medial temporal, and cerebellar regions from baseline to 1 month after completion of chemotherapy (M1). One year later (Y1), GMD had returned to baseline levels in some regions, though not all. No between-group differences were found at baseline, and changes were not seen in patients who did not receive chemotherapy or in healthy controls. These findings extended those of smaller, retrospective studies14–18 and demonstrated structural abnormalities related to chemotherapy in frontal brain regions, which underlie the cognitive functions most commonly found to be affected in studies of BC and treatment-related changes, including working memory (WM).19–21
Functional neuroimaging studies in BC to date have been cross- sectional studies and have shown increases and decreases in activation that vary by treatment stage, cognitive function studied, and comparison group.22–29 Here we examine BC- and treatment-related changes in brain activation and their course over time prospectively in patients treated with standard-dose chemotherapy (CTx+) or without chemotherapy (CTx−) and healthy controls. Prior cognitive studies have shown declines after chemotherapy, with improvement over time3,8,9,30,31 (although see also Wefel et al5 regarding later-onset cognitive changes and Kreukels et al32 and Weis et al33 regarding persistent cognitive deficits in a subgroup of individuals), and our recent finding of GMD changes followed the same pattern.13 We therefore hypothesized that chemotherapy-related activation changes in WM circuitry would be detectable at M1 and show partial to complete recovery at Y1. Although effects of antiestrogen treatment are less well characterized, we predicted that any changes due to these treatments alone would be detectable after treatment initiation but would not be expected to remit over the course of this study because treatment would be ongoing.
PATIENTS AND METHODS
Measures were completed at baseline (after surgery but before radiation, chemotherapy, and/or antiestrogen treatment), at M1, and at Y1, or at yoked intervals for CTx− and control groups.
Participants
Participants were female patients with BC—CTx+; n = 16 and CTx−; n = 12—and healthy controls (n = 15) drawn from the cohort described in McDonald et al.13 For this report, one CTx+ and two control participants were excluded because of scan artifacts, and one control was excluded because of outlying WM task performance (> three standard deviations below group mean). Patients had noninvasive (stage 0) or nonmetastatic invasive (stages I, II, or IIIA) disease and were treated with standard-dose chemotherapy regimens (Table 1). Exclusion criteria included prior cancer or cancer treatment and medical, neurologic, and psychiatric risk factors known to affect brain structure or function, as detailed in McDonald et al.13 Depressive symptoms were assessed by using the Center for Epidemiologic Studies-Depression Scale (CES-D),35 anxiety by using the State-Trait Anxiety Inventory-State subscale (STAI-S),36 and fatigue by using the Fatigue Symptom Inventory (FSI).37 Written informed consent was obtained according to the Declaration of Helsinki under a protocol approved by the Dartmouth College Committee for the Protection of Human Subjects.
Table 1.
Sample Demographic Characteristics
| Characteristic | CTx+ (n = 16) |
CTx− (n = 12) |
Control (n = 15) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | |
| Age at baseline, years | 52.9 | 8.6 | 52.7 | 7.2 | 50.5 | 6.0 | |||
| Years of education | 15.2 | 2.6 | 16.1 | 2.3 | 15.9 | 2.0 | |||
| Estimated full-scale IQ (Barona Index34) | 111.9 | 6.9 | 114.7 | 4.7 | 114.1 | 3.6 | |||
| Handedness | |||||||||
| Right | 14 | 12 | 13 | ||||||
| Left or ambidextrous | 2 | 0 | 2 | ||||||
| Days from baseline to M1 scan | 171.6 | 65.9 | 191.8 | 72.6 | 191.7 | 46.7 | |||
| Days from M1 to Y1 scan | 352.7 | 52.5 | 303.9 | 70.2 | 320.4 | 81.2 | |||
| Days from baseline to Y1 scan | 524.3 | 54.5 | 495.8 | 53.1 | 512.1 | 78.6 | |||
| Cancer stage | |||||||||
| 0 (DCIS)* | 0 | 4 | |||||||
| I | 4 | 6 | |||||||
| II | 11 | 2 | |||||||
| IIIA | 1 | 0 | |||||||
| Received radiotherapy | 11 | 10† | |||||||
| No. receiving antiestrogen therapy‡ | |||||||||
| Baseline | 0 | 1 TAM | |||||||
| M1 | 3 TAM | 6 TAM | |||||||
| 1 ANA | 1 TAM/ GOS | ||||||||
| 2 ANA | |||||||||
| Y1 | 9 TAM | 6 TAM | |||||||
| 1 ANA | 1 TAM/ GOS | ||||||||
| 2 LET | 2 ANA | ||||||||
| No. receiving psychotropic medication | |||||||||
| Baseline | 4 | 5 | |||||||
| M1 | 5 | 4 | |||||||
| Y1 | 5 | 6 | |||||||
| Chemotherapy regimen§ | |||||||||
| Doxorubicin/cyclophosphamide/paclitaxel | 11 | ||||||||
| Docetaxel/doxorubicin/cyclophosphamide | 2 | ||||||||
| Doxorubicin/cyclophosphamide | 3 | ||||||||
| Menstrual status∥ | |||||||||
| Menstruating throughout study | 0 | 4 | 7 | ||||||
| Postmenopausal (> 6 months) throughout study | 9 | 5 | 6 | ||||||
| Periods irregular at study entry, entered menopause (> 6 months) during study | 2 | 2 | 2 | ||||||
| Periods regular at study entry, entered menopause (> 6 months) during study | 4 | 1 | 0 | ||||||
Abbreviations: ANA, anastrozole; CTx+, patients treated with chemotherapy; CTx−, patients not treated with chemotherapy; DCIS, ductal carcinoma in situ; GOS, goserelin; IQ, intelligence quotient; LET, letrozole; M1, 1 month after completion of chemotherapy; SD, standard deviation; TAM, tamoxifen; Y1, 1 year after M1 visit.
Significant between-group difference, P = .01.
Eight received conventional local radiation; two received MammoSite baseline scans (3 and 36 days before scanning, respectively).
One CTx− participant began TAM 18 days before her baseline scan.
One CTx+ patient was treated with trastuzumab for 2 months after completion of chemotherapy.
Menstrual status was unknown for one CTx+ patient.
MRI Scan Acquisition
Scans were acquired on a 1.5T GE Signa LX scanner (GE Medical Systems, Waukesha, WI) with echospeed gradients and standard head coil. A gradient-echo, echo-planar sequence provided whole brain coverage for functional MRI (fMRI): repetition time (TR) = 2,500 ms, echo time (TE) = 40 ms, field of view (FoV) = 24 cm, and number of excitations (NEX) = 1, 29 interleaved 5-mm thick contiguous sagittal slices, yielding a 64 × 64 matrix with 3.75 mm2 in-plane resolution. Structural scans were acquired to rule out incidental pathology and for the previously reported GMD analyses13 (see Appendix [online only] for additional information regarding full scan protocol).
WM Task
As in our previous studies,24,38–44 a verbal n-back task was used. During scanning, participants heard a string of consonant letters (except L, W, and Y) presented one every 3 seconds. Task conditions were 0-, 1-, 2-, and 3-back, in a blocked design. For each consonant, participants used a button press device to signify whether the current letter was a match (ie, was the same as the designated target or the letter presented 1, 2, or 3 back in the sequence) or a nonmatch. Each condition was presented in 27-second epochs preceded by 3 seconds of instruction (eg, “the match is one back”). The four experimental conditions were each presented three times in pseudorandom order for a total of 12 task blocks (duration, 7 minutes, 47 seconds). Participants rehearsed a practice version of the task before scanning to ensure that they understood the demands of the task. Stimuli were presented through an MRI-compatible headphone system and programmed in Presentation, which recorded response accuracy and reaction times.
fMRI Image Analysis
Spatial realignment using a six-parameter model was performed on raw scan data by using Statistical Parametric Mapping, version 5 (SPM5; Wellcome Department of Cognitive Neurology, University College, London, United Kingdom). Realignment parameters were entered as covariates at the subject level, and volumes were normalized into Montreal Neurological Institute (MNI) space, resampled to 2 mm3 voxels, and smoothed to a full width at half maximum (FWHM) of 8 mm. Statistical parametric mapping on a voxel-by-voxel basis was conducted by using a general linear model approach. Contrast images comparing pairs of WM load conditions (eg, 3-back > 0-back) were created for each patient and were used in second-level multisubject/between-group analyses.
Random effects analyses were used for each contrast of interest to identify voxels where brain activation differed between groups and over time. Weighted contrast vectors were entered for cross-sectional between-group analyses, group-by-time interactions, and within-group longitudinal comparisons (as in McDonald et al13). For example, between-group differences at baseline were examined by entering values of 1 and −1 in respective group columns in the design matrix. The interaction for regions where CTx+ patients showed decreased activation compared with controls from baseline to M1 was modeled by entering 1 in the CTx+ baseline and control M1 columns and −1 in the CTx+ M1 and control baseline columns, respectively. Other interactions and alternate directionality were conducted in a similar fashion. For within-group analyses incorporating all three time-points, to model decreased activation from baseline to M1 followed by return to baseline levels at Y1, the vector 1 −2 1 was entered in the baseline, M1, and Y1 columns. For regions where declines from baseline to M1 persisted at Y1, a vector of 2 −1 −1 was entered in the same manner. The voxel-wise critical significance threshold (Pcrit) was set to .001, with a minimum cluster extent (k) of 10 voxels. The main effect of the contrast of interest (eg, 3-back > 0-back; P < .05; Appendix Fig A1, online only) for all groups at all time-points was included in the design matrix as an explicit mask. SPSS 19 (IBM Corporation, Somers NY) was used to analyze task performance and demographic differences (analysis of variance [ANOVA], general linear model, and χ2).
RESULTS
Demographic and Task Performance Data
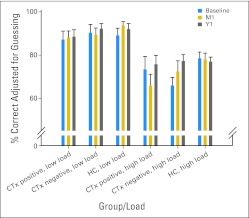
Consistent with typical treatment approaches, CTx+ patients had significantly higher stage disease than CTx− patients (χ2 = 11.29; 3 df; P = .01). There were no other between-group differences in demographics, menopausal status, or number of patients receiving psychotropic medications, or mood (CES-D), anxiety (STAI-S), or fatigue (FSI composite) self-ratings at any time-point or any group-by-time interactions (P > .05). CES-D and STAI-S group means were well below clinically significant levels (Appendix Table A1, online only). FSI composite means were mildly increased for all three groups, with 33% to 63% of participants endorsing clinically meaningful fatigue45 during the study (Appendix Table A1). Task performance accuracy and reaction times did not differ between groups or over time for individual task conditions (Appendix Table A2, online only). On the basis of our previous studies demonstrating greater sensitivity to group differences at higher WM loads,38–44 performance accuracy at high load (2-back, 3-back) was compared with that at low load (0-back, 1-back). Repeated measures ANOVA showed the expected main effect of load (P < .001; performance was better for low load). The profile of the CTx+ group showed a nonsignificant (P = .16) decrease in high-load performance at M1 compared with baseline, with improved performance at Y1. This pattern was not apparent for any other group (Fig 1).
Fig 1.
N-back performance data. Task performance data by group for low (0-back and 1-back) and high (2-back and 3-back) working memory load. Note pattern of decreased performance at 1 month after chemotherapy completion (M1) or yoked interval with recovery at 1 year after M1 visit (Y1) in patients with breast cancer treated with chemotherapy (CTx positive). CTx negative, patients not treated with chemotherapy; HC, healthy control.
Imaging Analyses
To reduce the number of contrasts, and considering that the greatest impact of chemotherapy on performance was at higher WM load, imaging analyses focused on the highest WM load condition (3-back > 0-back). A similar pattern of findings was apparent for 2-back > 0-back, although statistical significance was attenuated. We also examined results by using disease stage as a covariate, with a similar overall pattern of findings. MNI coordinates, cluster extents, T and Z scores, and region descriptions for all analyses are presented in Table 2.
Table 2.
Regional Task-Related Activation Changes 3-Back > 0-Back
| Contrast of Interest | Presence/Absence of Significant Clusters | MNI Coordinates |
Cluster Extent (k) | T Score | Z Score | Region Description (for cluster peak) | BA | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Between-group analyses | |||||||||
| Baseline | |||||||||
| CTx+ > HCs | 20 | −74 | 38 | 28 | 4.03 | 3.89 | Right precuneus | 7 | |
| 42 | 34 | 16 | 73 | 3.90 | 3.78 | Right MFG | 46 | ||
| 46 | −66 | 48 | 11 | 3.72 | 3.61 | Right IPL | 40 | ||
| CTx− > HCs | 20 | −72 | 38 | 115 | 5.23 | 4.96 | Right precuneus | 7 | |
| −42 | 26 | 12 | 23 | 3.77 | 3.66 | Left IFG | 13 | ||
| HCs > CTx+ | −16 | −70 | 56 | 57 | 4.10 | 3.96 | Left SPL | 7 | |
| HCs > CTx− | −14 | −62 | 56 | 54 | 4.07 | 3.93 | Left precuneus | 7 | |
| CTx+ > CTx− | 52 | 12 | 24 | 62 | 4.31 | 4.15 | Right IFG | 44 | |
| CTx− > CTx+ | No significant clusters | ||||||||
| M1 | |||||||||
| CTx+ > HCs | No significant clusters | ||||||||
| CTx− > HCs | No significant clusters | ||||||||
| HCs > CTx+ | No significant clusters | −16 | −72 | 56 | 34 | 3.65 | 3.55 | Left SPL | 7 |
| HCs > CTx− | No significant clusters | ||||||||
| CTx+ > CTx− | No significant clusters | ||||||||
| CTx− > CTx+ | No significant clusters | ||||||||
| Y1 | |||||||||
| CTx+ > HCs | −44 | 48 | 8 | 28 | 4.05 | 3.91 | Left MFG | 10 | |
| CTx− > HCs | −42 | 46 | 6 | 71 | 4.29 | 4.13 | Left IFG | 10 | |
| −48 | 22 | 38 | 19 | 3.82 | 3.71 | Left MFG | 9 | ||
| −30 | −80 | 44 | 17 | 3.57 | 3.47 | Left SPL | 7 | ||
| HCs > CTx+ | −12 | −60 | 54 | 30 | 4.33 | 4.17 | Left precuneus | 7 | |
| HCs > CTx− | No significant clusters | ||||||||
| CTx+ > CTx− | 44 | 10 | 50 | 29 | 4.02 | 3.88 | Right MFG | 6 | |
| 54 | 10 | 22 | 22 | 3.81 | 3.70 | Right IFG | 44 | ||
| −44 | 14 | 54 | 12 | 3.64 | 3.54 | Left MFG | 6 | ||
| CTx− > CTx+ | No significant clusters | ||||||||
| Group-by-time interaction analyses | |||||||||
| HCs > CTx+ from baseline to M1 | −38 | 44 | 6 | 40 | 3.82 | 3.70 | Left IFG | ||
| HCs > CTx− from baseline to M1 | −42 | 24 | 10 | 24 | 3.88 | 3.76 | Left IFG | 13 | |
| CTx+ > HCs from baseline to M1 | −58 | −50 | −14 | 19 | 4.00 | 3.87 | Left MTG | 37 | |
| −6 | −20 | 8 | 10 | 3.47 | 3.38 | Left thalamus | |||
| CTx+ > CTx− from baseline to M1 | 18 | −90 | −28 | 19 | 3.73 | 3.62 | Right cerebellum | ||
| −48 | 12 | 6 | 14 | 3.68 | 3.58 | Left PCG | 44 | ||
| −60 | −48 | −12 | 11 | 3.67 | 3.56 | Left MTG | 37 | ||
| CTx− > HCs from baseline to M1 | No significant clusters | ||||||||
| CTx− > CTx+ from baseline to M1 | No significant clusters | ||||||||
| Within-group analyses | |||||||||
| CTx+ decreased activation from baseline to M1 | −10 | 26 | 34 | 10 | 3.68 | 3.58 | Left MeFG | 9 | |
| −38 | 44 | 6 | 12 | 3.55 | 3.46 | Left IFG | |||
| CTx− decreased activation from baseline to M1 | −42 | −74 | −26 | 26 | 4.79 | 4.58 | Left cerebellum | ||
| −50 | 44 | −8 | 14 | 3.86 | 3.74 | Left MFG | 47 | ||
| −42 | 22 | 12 | 17 | 3.69 | 3.59 | Left IFG | 13 | ||
| HCs decreased activation from baseline to M1 | No significant clusters | ||||||||
| CTx+ decreased activation from baseline to M1 with return to baseline at Y1 | −38 | 44 | 6 | 89 | 4.43 | 4.26 | Left IFG | ||
| −10 | 26 | 34 | 16 | 4.01 | 3.88 | Left MeFG | 9 | ||
| CTx− decreased activation from baseline to M1 with return to baseline at Y1 | −42 | −74 | −26 | 33 | 5.05 | 4.80 | Left cerebellum | ||
| CTx+ decreased activation from baseline to M1 with persistent decrease at Y1 | 44 | 56 | −4 | 11 | 3.67 | 3.57 | Right MFG | 10 | |
| CTx− decreased activation from baseline to M1 with persistent decrease at Y1 | −50 | 44 | −8 | 11 | 3.75 | 3.64 | Left MFG | 47 | |
Abbreviations: BA, Brodmann area; CTx+, patients treated with chemotherapy; CTx−, patients not treated with chemotherapy; HC, healthy control; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; M1, 1 month after completion of chemotherapy; MeFG, medial frontal gyrus; MFG, middle frontal gyrus; MNI, Montreal Neurological Institute; MTG, middle temporal gyrus; PCG, precentral gyrus; SPL, superior parietal lobule; Y1, 1 year after M1 visit.
Cross-Sectional Group Comparisons
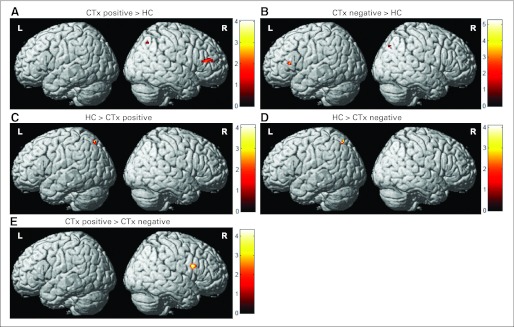
At baseline, patients showed significantly increased bifrontal and right parietal activation compared with controls (Figs 2A and 2B). CTx+ patients also showed greater right inferior frontal activation compared with CTx− patients (Fig 2E). At M1, this hyperactivation was absent; however, by Y1, left frontal hyperactivation compared with controls was again apparent in both cancer groups, with CTx− patients also showing greater left parietal activation than controls (Table 2). At Y1, CTx+ patients again showed greater frontal activation than CTx− patients, including regions within bilateral middle frontal gyri in addition to left inferior frontal gyrus (Table 2). Controls showed greater left parietal activation than CTx− patients at baseline and CTx+ patients at all time-points (Figs 2C and 2D; Table 2).
Fig 2.
Baseline between-group differences in activation (3-back > 0-back contrast). Before adjuvant treatment, patients with breast cancer showed (A, B) hyperactivation of frontal regions bilaterally and (C, D) decreased left parietal activation compared with healthy controls (HCs), and patients treated with chemotherapy (CTx positive) showed (E) greater right frontal activation than those who did not receive chemotherapy (CTx negative; Pcrit [voxel-wise critical significance threshold] = .001; cluster extent [k] = 10).
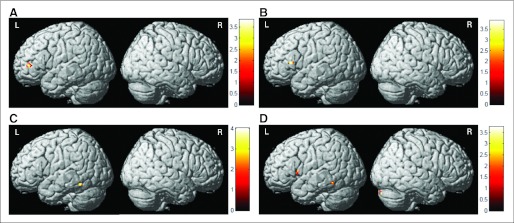
Group-By-Time Interactions
Compared with controls, both cancer groups demonstrated decreased left inferior frontal activation at M1 compared with baseline (Figs 3A and 3B). CTx+ patients also showed increased left thalamic and posterior middle temporal gyrus activation compared with controls in this interval (Fig 3C) and increased right cerebellar and left inferior precentral and posterior middle temporal gyrus activation compared with the CTx− group (Fig 3D).
Fig 3.
Interaction analyses showing between-group differences in brain activation from baseline to 1 month after completion of chemotherapy (M1; 3-back > 0-back contrast). (A) Decreased activation from baseline to M1 in patients with breast cancer treated with chemotherapy (CTx positive) compared with healthy controls (HCs). (B) Decreased activation from baseline to M1 in patients not receiving chemotherapy (CTx negative) compared with HCs. (C) Increased activation from baseline to M1 in CTx-positive patients compared with HCs. (D) Increased activation from baseline to M1 in CTx-positive patients compared with CTx-negative patients (Pcrit [voxel-wise critical significance threshold] = .001; cluster extent [k] = 10).
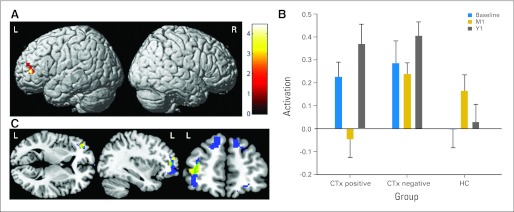
Within-Group Comparisons
Both cancer groups showed decreased left frontal activation from baseline to M1, with CTx− patients also showing decreased left cerebellar activation (Table 2). There were no regions where controls showed decreased activation from baseline to M1. Differing patterns of change at Y1 were observed for the cancer groups. For CTx+ patients, this decreased left frontal activation from baseline to M1 showed a return to baseline levels at Y1 (Figs 4A and 4B). This is the same region where interaction analyses showed significant decline from baseline to M1 in CTx+ patients compared with controls (Fig 3A). In CTx− patients, decreased activation from baseline to M1 with return to baseline levels at Y1 was apparent in the left cerebellum (Table 2). Both cancer groups also showed decreased middle frontal gyrus activation from baseline to M1, which remained decreased at Y1 (Table 2).
Fig 4.
Alterations in brain activation over time (3-back > 0-back contrast). (A) Brain activation decrease in patients with breast cancer treated with chemotherapy (CTx positive) from baseline to 1 month after chemotherapy completion (M1) with return to baseline levels of hyperactivation at 1 year after M1 visit (Y1), displayed over atlas template. (B) Activation pattern at left frontal peak displayed in (A) shown graphically for all groups at all time-points (MNI coordinates −38, 44, 6; cluster means extracted by using MarsBaR version 0.4246). These frontal changes in CTx-positive patients overlapped with regions of gray matter change following the same pattern of change over time13 as illustrated in (C), in which brain activation change is shown in yellow, gray matter change is shown in blue, and the intersection of activation and gray matter change is shown in green (displayed by using MRIcroGL [http://www.cabiatl.com/mricrogl/]; Pcrit [voxel-wise critical significance threshold] = .001; cluster extent [k] = 10). CTx negative, not treated with chemotherapy; HC, healthy control.
Examination of the relationship between frontal activation changes and the GMD changes we previously reported in CTx+ patients13 demonstrated that left inferior frontal gyrus shows the same pattern of decreased GMD/WM-related activation from baseline to M1 with return to baseline GMD/activation at Y1 in both analyses (Fig 4C). Comparison with the overall main effect of activation (Appendix Fig A1, online only) demonstrates that regions of altered activation in the cancer groups were generally within typical WM circuitry or in adjacent frontal or parietal regions.
DISCUSSION
We used a prospective, longitudinal approach and found alterations in cognitive task-related brain activation related to BC and its treatment. Our finding of WM-related frontal hyperactivation before adjuvant treatment in patients compared with controls extends prior pretreatment research showing this pattern22,28 by demonstrating that this abnormality persists over time in the first 2 years postdiagnosis. Taken together, the results of our study and those of the prior baseline reports suggest that even before adjuvant treatment, patients with BC are engaging in compensatory hyperactivation of brain circuitry to support WM functioning in response to as yet unknown effects of the cancer disease process. Our findings are also consistent with those of Silverman et al,29 who used oxygen-15 positron emission tomography to study BC survivors who received chemotherapy compared with women who had not received systemic therapy, about half of whom were BC survivors. During short-term memory processing, CTx+ patients showed increased superior and inferior frontal lobe activation, although the comparison group showed greatest increase in parietal regions. These are essentially the same frontal and parietal regions where we found increased and decreased activation, respectively, in patients compared with controls. Using the same fMRI task as in this study, we also observed increased WM circuitry activation, particularly in the frontal lobes, in a patient with BC treated with chemotherapy and tamoxifen relative to her identical twin, who had no cancer history.24
In contrast, de Ruiter et al27 used an fMRI measure of planning and problem-solving and found decreased bifrontal and biparietal activation in CTx+ BC survivors post-treatment with standard-dose and high-dose chemotherapy, stem-cell transplantation, and tamoxifen compared with those who did not receive systemic treatment. These decreases were subsequently shown to be related to decreased gray matter volume and white matter integrity.47 Kesler et al26 also showed reduced left frontal activation in CTx+ and CTx− BC survivors compared with controls during an fMRI measure of reasoning and problem solving. CTx+ patients showed additional reduced left frontal activation compared with both other groups, which was related to objective and subjective executive functioning. Our cohort was closer to treatment and approximately 5 years younger on average than patients in these retrospective studies. Our finding of greater activation in CTx+ patients compared with CTx− patients at Y1 in some of the same frontal regions may therefore offer additional insight into the evolution of these functional changes over time and/or their relationship to cognitive aging. Patients with BC may lose the ability to compensate functionally with aging and so may show decreased activation over time. A similar pattern has been reported in Alzheimer's disease and its precursors,48 highlighting the need for further study of the interaction of the effects of cancer and cognitive aging.
After initiation of chemotherapy or antiestrogen treatment, both cancer groups showed decreases in frontal lobe activation compared with controls. Within-group analyses showed that decreased frontal activation at M1 was followed by return to activation approximating baseline levels of hyperactivation at Y1 for some regions, although other frontal areas showed decreased activation at M1 that remained decreased at Y1. In the CTx+ group, this brain activation change was accompanied by a nonsignificant pattern of declining task performance at M1 followed by improvement at Y1. For both cancer groups, baseline activation differences compared with controls were attenuated following treatment initiation (M1), but they reemerged at Y1. Within the CTx+ group, frontal lobe activation changes were apparent in the same regions where we found alterations in GMD13 and showed the same pattern of change over time (decreased activation/GMD at M1 compared with baseline, with partial return to baseline levels at Y1). CTx− patients (75% of whom received antiestrogen treatment) also showed alterations in brain activation in this study, although these individuals did not show changes in GMD, potentially demonstrating differential treatment effects. These structural and functional changes suggest greater vulnerability of frontal regions to treatment effects, particularly chemotherapy. Examination of biologic factors that may contribute to these apparent regional differences will be important in future research in this area. Although psychosocial factors such as mood, anxiety, and fatigue may also play a role in subjective and objective cognitive changes related to cancer and its treatment, the lack of between-group differences in these factors suggests they do not account for our findings.
Our data provide new information on time course for these functional changes, demonstrating decreased activation compared with baseline following completion of chemotherapy and initiation of antiestrogen treatment. Changes are greatest in inferior frontal regions that colocalize with alterations in gray and white matter post-treatment in longitudinal studies.13,49 The hyperactivation apparent in patients with cancer at baseline and Y1 suggests the need for compensatory recruitment of an expanded spatial extent of neural circuitry to support WM functioning. At M1, CTx+ patients are unable to maintain this hyperactivation, presumably as a result of impairment in brain function due to chemotherapy. These functional changes mirror previous studies demonstrating the greatest cognitive difficulty shortly after cancer treatment but improvement over time, suggesting that the observed alterations in brain activation may reflect an inability to effectively compensate functionally which improves to some degree with time, as reflected by return to the baseline state of hyperactivation compared with controls. Overall, our pattern of findings is consistent with that in the literature7–11 demonstrating alterations in cognitive functioning differentially related to chemotherapy and antiestrogen treatment.
Limitations of the overall study design related to sample characteristics, power, and covariates were included in our prior report.13 In the context of fMRI, an additional consideration is model testing. Specific hypothesized models of change were evaluated but were not exhaustive of all possible permutations of change over time. Subgroups of patients (eg, those who experience persistent cognitive changes that do not improve over time32,33) may show different trajectories of activation change from those examined here. Similarly, changes in hormonal (menstrual) status may also play a role in these results (eg, chemotherapy-induced ovarian failure). In our cohort, six of 16 CTx+ patients experienced menopause presumably due to chemotherapy. By comparison, three of 12 CTx− patients and two of 15 controls experienced menopause during the course of the study. Although this ratio was not significantly different between groups, changes in hormonal status have been shown to affect brain activation.50–59 This study was not powered for such subgroup analyses, which would optimally be conducted in a larger cohort, perhaps via multicenter collaborations.
Our findings suggest that cognitive and brain changes in patients with BC are multifactorial in nature, evolve over time, and are not simply attributable to chemotherapy. Future work should consider aspects of the cancer disease process in addition to superimposed effects of chemotherapy and antiestrogen treatments, while also taking into account variables such as timing of assessments in relation to treatment. There is initial evidence that cognitive changes after chemotherapy are associated with genetic variation,60,61 with one study61 specifically highlighting the potential interaction between genetic variation, cancer and its treatment, cognition, and frontal lobe functioning. Improved understanding of risk factors will be critical to identifying vulnerable patients and developing preventative and treatment approaches.
Acknowledgment
We thank Charlotte Furstenberg, Leigh Chesnut, Susan Horrigan, Carrie Kruck, Vivian Horovitch-Kelley, Peter Kaufman, MD, Gary Schwartz, MD, and Alexander Mamourian, MD, for their assistance. We are grateful to our participants for their time and effort; this research would not have been possible without their willingness to participate during a challenging time in their lives.
Appendix
Full-Scan Protocol
Other scan sequences beyond those described in this article were also acquired during the scan session. Because this article focused on the working memory task analyses, we elected not to list all series acquired. However, in brief, the scan session included T1, proton density/T2, and fluid attenuated inversion recovery (FLAIR) structural sequences, working and episodic memory functional magnetic resonance imaging (fMRI) tasks, and diffusion tensor imaging. The T1 and working memory tasks were the first two series acquired in the scan session, which lasted about 90 minutes in total. Further scan acquisition information is available from the corresponding authors.
Fig A1.
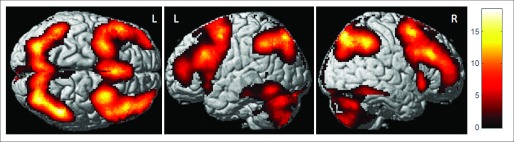
Mean activation map for 3-back > 0-back for all groups at all time-points showing the typical working memory activation pattern, including bilateral frontal, parietal, and cerebellar regions (Pcrit [voxel-wise critical significance threshold] = .05). This map was used as an explicit mask for all subsequent imaging analyses.
Table A1.
Mood, Anxiety, and Fatigue Self-Rating Raw Scores*
| Time-Point | Measure | CTx+ (n = 16) |
CTx− (n = 12) |
Control (n = 15) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. Above Threshold* | Mean | SD | No. Above Threshold* | Mean | SD | No. Above Threshold* | ||
| Baseline | CES-D | 8.7 | 8.2 | 2 | 5.7 | 6.6 | 1 | 4.9 | 4.7 | 0 |
| STAI-S | 30.5 | 10.7 | 2 | 28.8 | 13.1 | 1 | 25.9 | 7.1 | 0 | |
| FSI | 3.2 | 2.0 | 8 | 3.2 | 1.7 | 7 | 3.3 | 1.8 | 9 | |
| M1 | CES-D | 10.4 | 10.4 | 3 | 6.1 | 5.7 | 1 | 4.6 | 4.9 | 0 |
| STAI-S | 31.5 | 13.9 | 2 | 27.1 | 9.2 | 1 | 27.4 | 8.9 | 1 | |
| FSI | 3.7 | 2.1 | 10 | 2.9 | 1.8 | 7 | 2.7 | 1.4 | 5 | |
| Y1 | CES-D | 6.6 | 6.0 | 3 | 7.5 | 10.4 | 1 | 5.2 | 9.7 | 1 |
| STAI-S | 27.6 | 9.1 | 1 | 28.3 | 11.3 | 1 | 26.4 | 7.7 | 0 | |
| FSI | 3.0 | 2.2 | 6 | 3.4 | 1.7 | 7 | 2.4 | 1.3 | 5 | |
NOTE. There were no significant between-group differences at any time-point or any group-by-time interactions (P > .05).
Abbreviations: CES-D, Center for Epidemiologic Studies-Depression Scale (raw score); CTx+, patients treated with chemotherapy; CTx−, patients not treated with chemotherapy; FSI, Fatigue Symptom Inventory (composite raw score); M1, 1 month after completion of chemotherapy; SD, standard deviation; STAI-S, State-Trait Anxiety Inventory-State Scale (raw score); Y1, 1 year after M1 visit.
Thresholds for significant symptomatology: CES-D raw score ≥ 16, STAI-S T score ≥ 65, FSI composite raw score ≥ 3.
Table A2.
N-Back Task Performance
| Time-Point | Measure | CTx+ (n = 16) |
CTx− (n = 12) |
Control (n = 15) |
|||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Baseline | 0-back | 96.1 | 8.2 | 97.2 | 5.6 | 91.4 | 17.9 |
| 1-back | 78.3 | 30.1 | 83.5 | 24.8 | 86.9 | 14.1 | |
| 2-back | 80.1 | 27.5 | 75.1 | 17.8 | 83.2 | 16.0 | |
| 3-back | 66.7 | 25.2 | 56.8 | 20.2 | 73.8 | 12.3 | |
| M1 | 0-back | 92.8 | 11.9 | 93.6 | 11.2 | 93.3 | 10.6 |
| 1-back | 83.4 | 23.1 | 85.2 | 17.0 | 94.2 | 9.1 | |
| 2-back | 71.7 | 27.7 | 83.0 | 12.9 | 88.3 | 11.1 | |
| 3-back | 60.1 | 26.2 | 62.0 | 23.3 | 67.6 | 18.9 | |
| Y1 | 0-back | 88.0 | 21.9 | 94.0 | 11.3 | 90.1 | 14.5 |
| 1-back | 89.1 | 13.2 | 90.4 | 11.4 | 93.9 | 14.0 | |
| 2-back | 83.1 | 16.1 | 82.1 | 13.5 | 78.0 | 12.4 | |
| 3-back | 68.4 | 22.9 | 72.4 | 16.8 | 76.0 | 9.6 | |
Abbreviations: CTx+, patients treated with chemotherapy; CTx−, patients not treated with chemotherapy; SD, standard deviation.
Footnotes
Supported by Grants No. R01 CA101318, R01 CA087845, and R25 CA117865 from the National Cancer Institute, National Institutes of Health, and Grant No. 87884 from the Indiana Economic Development Corporation.
Presented at the Fourth Annual International Neuropsychological Society Conference, Boston, MA, February 1-4, 2006; the 13th Annual Meeting of the Organization for Human Brain Mapping, Chicago, IL, June 10-14, 2007; and the International Cognition and Cancer Task Force, New York, NY, March 8-9, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Brenna C. McDonald, Tim A. Ahles, Andrew J. Saykin
Financial support: Tim A. Ahles, Andrew J. Saykin
Administrative support: Brenna C. McDonald, Tim A. Ahles, Andrew J. Saykin
Provision of study materials or patients: Tim A. Ahles, Andrew J. Saykin
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 3.Jansen CE, Cooper BA, Dodd MJ, et al. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 4.Wefel JS, Lenzi R, Theriault R, et al. ‘Chemobrain' in breast carcinoma?: A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal K, Onami S, Mortimer JE, et al. Cognitive changes associated with endocrine therapy for breast cancer. Maturitas. 2010;67:209–214. doi: 10.1016/j.maturitas.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 10.Jim HS, Donovan KA, Small BJ, et al. Cognitive functioning in breast cancer survivors: A controlled comparison. Cancer. 2009;115:1776–1783. doi: 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quesnel C, Savard J, Ivers H, et al. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 12.Vardy J, Wefel JS, Ahles T, et al. Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 13.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakamata Y, Matsuoka Y, Inagaki M, et al. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci Res. 2007;59:383–389. doi: 10.1016/j.neures.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 16.McDonald BC, Saykin AJ, Ahles TA. Brain imaging investigation of chemotherapy-induced neurocognitive changes, in Meyers CA, Perry JR (eds): Cognition and Cancer. Cambridge, MA: Cambridge University Press; 2008. pp. 19–32. [Google Scholar]
- 17.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: Neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- 18.Yoshikawa E, Matsuoka Y, Yamasue H, et al. Prefrontal cortex and amygdala volume in first minor or major depressive episode after cancer diagnosis. Biol Psychiatry. 2006;59:707–712. doi: 10.1016/j.biopsych.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Anderson-Hanley C, Sherman ML, Riggs R, et al. Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 20.Jansen CE, Miaskowski C, Dodd M, et al. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104:2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 21.Stewart A, Bielajew C, Collins B, et al. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006;20:76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 22.Cimprich B, Reuter-Lorenz P, Nelson J, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 23.Eberling JL, Wu C, Tong-Turnbeaugh R, et al. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson RJ, McDonald BC, Saykin AJ, et al. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesler SR, Bennett FC, Mahaffey ML, et al. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15:6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesler SR, Kent JS, O'Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherling C, Collins B, Mackenzie J, et al. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An FMRI study. Front Hum Neurosci. 2011;5:122. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schagen SB, Muller MJ, Boogerd W, et al. Late effects of adjuvant chemotherapy on cognitive function: A follow-up study in breast cancer patients. Ann Oncol. 2002;13:1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- 32.Kreukels BP, van Dam FS, Ridderinkhof KR, et al. Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin Breast Cancer. 2008;8:80–87. doi: 10.3816/CBC.2008.n.006. [DOI] [PubMed] [Google Scholar]
- 33.Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: ‘Subjective' complaints and ‘objective' neuropsychological test results. Psychooncology. 2009;18:775–782. doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- 34.Barona A, Reynolds CR, Chastain R. A demographically based index of premorbid intelligence for the WAIS-R. J Consult Clin Psychol. 1984;52:885–887. [Google Scholar]
- 35.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Spielberger CD. State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 37.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 38.Dumas JA, Saykin AJ, McDonald BC, et al. Nicotinic versus muscarinic blockade alters verbal working memory-related brain activity in older women. Am J Geriatr Psychiatry. 2008;16:272–282. doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAllister TW, Saykin AJ, Flashman LA, et al. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- 40.McAllister TW, Sparling MB, Flashman LA, et al. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- 41.McAllister TW, Flashman LA, McDonald BC, et al. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 42.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 43.Wishart HA, Saykin AJ, McDonald BC, et al. Brain activation patterns associated with working memory in relapsing-remitting MS. Neurology. 2004;62:234–238. doi: 10.1212/01.wnl.0000103238.91536.5f. [DOI] [PubMed] [Google Scholar]
- 44.Wishart HA, Saykin AJ, Rabin LA, et al. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry. 2006;163:1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- 45.Donovan KA, Jacobsen PB, Small BJ, et al. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage. 2008;36:480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brett M, Anton JL, Valabregue R, et al. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2-6, 2002; Sendai, Japan. (abstr 497) [Google Scholar]
- 47.de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. doi: 10.1002/hbm.21422. [epub ahead of print on September 23, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 50.Berman KF, Schmidt PJ, Rubinow DR, et al. Modulation of cognition-specific cortical activity by gonadal steroids: A positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig MC, Fletcher PC, Daly EM, et al. Gonadotropin hormone releasing hormone agonists alter prefrontal function during verbal encoding in young women. Psychoneuroendocrinology. 2007;32:1116–1127. doi: 10.1016/j.psyneuen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Craig MC, Fletcher PC, Daly EM, et al. A study of visuospatial working memory pre- and post-Gonadotropin Hormone Releasing Hormone agonists (GnRHa) in young women. Horm Behav. 2008;54:47–59. doi: 10.1016/j.yhbeh.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Craig MC, Fletcher PC, Daly EM, et al. Physiological variation in estradiol and brain function: A functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav. 2008;53:503–508. doi: 10.1016/j.yhbeh.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich T, Krings T, Neulen J, et al. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. Neuroimage. 2001;13:425–432. doi: 10.1006/nimg.2001.0703. [DOI] [PubMed] [Google Scholar]
- 55.Gleason CE, Schmitz TW, Hess T, et al. Hormone effects on fMRI and cognitive measures of encoding: Importance of hormone preparation. Neurology. 2006;67:2039–2041. doi: 10.1212/01.wnl.0000247277.81400.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 57.Persad CC, Zubieta JK, Love T, et al. Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Fertil Steril. 2009;92:197–204. doi: 10.1016/j.fertnstert.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Effect of estrogen on brain activation patterns of postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 59.Smith YR, Love T, Persad CC, et al. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91:4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 61.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]