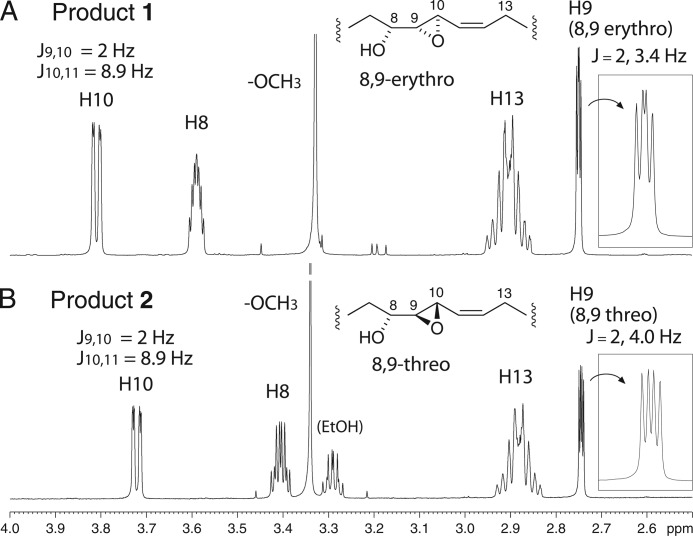
FIGURE 3.
Partial 1H NMR spectra of products 1 and 2, 8R-hydroxy-9,10-epoxy-20:3 diastereomers. The inset (right side) on each spectrum shows an expanded view of H9. The 2-Hz coupling for J9,10 establishes the trans-epoxide configuration in both 1 and 2. The more downfield chemical shift of H8 in 1 and the 3.4- and 4.0-Hz couplings for J8,9 support the erythro and threo assignments for 1 and 2, respectively (see main text) (20).