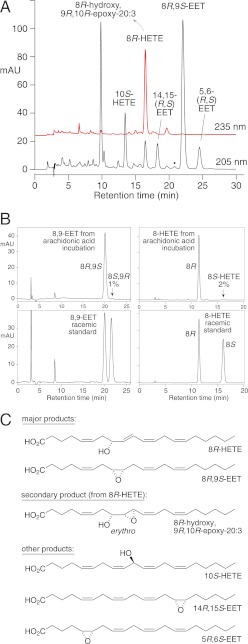
FIGURE 6.
Products formed from arachidonic acid by cAOS/PhIO. A, RP-HPLC analysis of an aliquot using a Thomson Instrument Co. TLC-Advantage ODS column (25 × 0.46 cm), a solvent of MeOH/H2O/HAc (80/20/0.01 by volume), and a flow rate of 1 ml/min, with on-line UV detection (Agilent 1100 series diode array detector) at 205 and 235 nm. The black dot at ∼21 min marks the expected retention time of 11,12-EET (not present). mAU, milliabsorbance units. B, chiral analysis of 8,9-EET (left panels) and 8-HETE (right panels) formed by cAOS/PhIO from arachidonic acid. 8,9-EET was analyzed as the free acid using a Chiralpak AD column (25 × 0.46 cm) and a solvent of hexane/methanol/glacial acetic acid (100:2:0.05) at a flow rate of 1 ml/min with UV detection at 205 nm. 8-HETE was analyzed as the methyl ester using the same conditions except with a solvent of hexane/methanol (100:2, v/v) with UV detection at 235 nm. C, structures of the products. The supplemental material illustrates chiral analysis of 5,6-EET (supplemental Fig. S7), 14,15-EET (supplemental Fig. S8), and 10-HETE (supplemental Fig. S9).