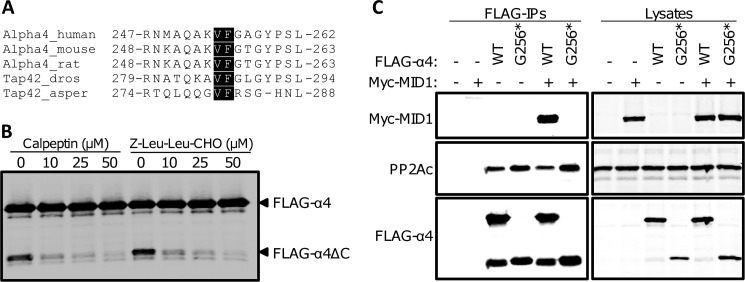
FIGURE 4.
Calpain-mediated cleavage of α4 results in an N-terminal fragment that binds PP2Ac but not MID1. A, sequence alignment of the region within multiple species of α4 that encompasses the calpain cleavage site; the conserved VF residues are highlighted. B, HEK293FT cells expressing wild type FLAG-α4 were treated with increasing concentrations of calpeptin or Z-Leu-Leu-CHO for 4 h prior to lysis. Cell lysates were subjected to Western analysis using an α4 antibody. C, HEK293FT cells were transfected with wild type FLAG-α4 WT and Gly256* alone or together with (+) or without (−) Myc-MID1. FLAG-tagged proteins were immunoprecipitated (FLAG-IPs) from the cell lysates and analyzed by Western using the Myc, PP2Ac, and α4 antibodies. The cell lysates were analyzed similarly. asper, Aspergillus; dros, Drosophila.