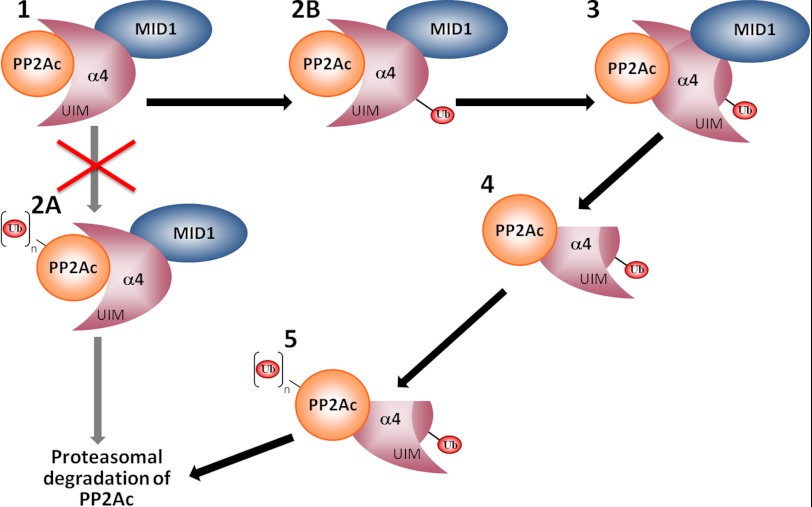
FIGURE 6.
Working model for MID1/α4 regulation of PP2Ac. MID1, α4, and PP2Ac form a ternary complex (1) in cells. Previous studies have suggested that α4 facilitates MID1-dependent polyubiquitination of PP2Ac (2A) and subsequent proteasomal degradation; however, our findings challenge this paradigm and support a model in which MID1 serves as the E3 ligase for α4 (2B), leading to a conformational change in α4 whereby the UIM of α4 binds in cis to the covalently attached ubiquitin (Ub; 3). This structural rearrangement then leads to calpain-mediated cleavage of the C terminus of α4 (4), allowing for polyubiquitination of PP2Ac by a currently unknown E3 ligase (5) and subsequent degradation by the proteasome.